ABDA-Database German SMPC Drug Dictionary in a nutshell
WHAT DOES SMPC STAND FOR?
The Summary of Product Characteristics (SPC or SmPC) is a specific document required within the European Commission before any medicinal product or biocidal product is authorised for marketing.
ABDA DATABASE GERMAN SMPC DRUG DICTIONARY
The ABDA Database German SmPC Drug Dictionary lists comprehensive information on original pharmaceutical products and generics available in Germany, Switzerland, Austria and other countries.
- check out all the structured data on Marketing Authorisation Holders´ (MAH) contact details in Germany
- check out the Marketing Authorisation Holders of more than 58.000 registered medicinal products in Germany
- Including active ingredients and their pharmacological and toxicological information, Clinical Particulars such as Interactions, Adverse Reactions, and Contra-Indications
- The Marketing Authorisation Holders Drug Dictionaries are updated twice a month
Suitable for:
Pharma Industry, Pharma Trade, Health Insurance, Regulatory
Source: ABDATA PHARMA-DATA-SERVICE
Eschborn, Germany
Related subscription
This database can be accessed after purchasing the subscription package ‘Drug Dictionaries’ or ‘Premium’
What customers say
For me as Contract Research Organisation (CRO) the Marketing Authorisation Holder Drug Dictionaries are a big help to find SmPCs of the different MAHs in the authorised markets.
What is practical is that you can find ALL the articles that are sold in the pharmacy in one portal: Medicines on the market, medical products, aids etc. on the market, medicines withdrawn from the market, foreign medicines etc..
We use the ABDA-Database German SMPC Drug Dictionary Medicines and International Medicines to offer our customers, in particular pharmacies and hospitals, international procurement opportunities for locally unavailable medicines (in Germany, for example, individual imports in accordance with §73.3 AMG). Thank you very much for your fast and good service!
Background information
List of content provided in the ABDA database German SMPC Drug Dictionary
ABDA Database – Medicinal Products
Composition
active ingredients including amount, additives
Classifications
- ABDATA-indications
- ATC-Code (German and WHO)
Structure of dosage form
The dosage forms are allocated to certain structures. Therefore, medicinal products can be combined to integrative groups according to certain criteria. It is being differentiated between the aspect „what is within the package? “and structures of application „how is the drug to be applied?“
Storage and durability after opening
structured data for storage and durability after opening or preparation of a medicinal product
Standard advice sentences
- application advices and dosage
- additives
- pregnancy
- lactation period
Monograph to finished dosage pharmaceutical products
comprehensive texts inform on
- indication
- contraindication
- application advices and dosage
- side effects
- characteristics
- stability period and storage
Structure of the information within the SmPC
What is not included in the EMA SmPC?
Detailed information on the scientific development, which is available in
the public assessment report
- Information in non-approved indication
- Because the MAH has not claimed the indication
- An indication has been claimed but data did not demonstrate a positive benefit risk of the medicine; withdrawal or refusal AR provide available data.
- Exception in the paediatric group; the Paediatric Regulation aims to improve the information regarding this subgroup by providing all information on clinically relevant trials
- Specific issue for which data is lacking
- General advice on the treatment of particular medical conditions
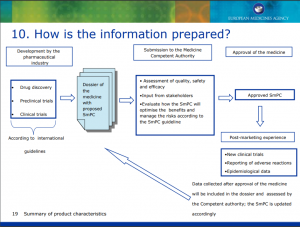
How is the information prepared?
Where to find more information?
- European Medicines Agency
http://www.ema.europa.eu
- Eudrapharm
http://www.eudrapharm.eu
- SmPC guideline http://ec.europa.eu/health/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf
- Information on benefit-risk of medicines: patients’, consumers’ and healthcare professionals’ expectations http://www.ema.europa.eu/docs/en_GB/document_library/Other/2009/12/WC500018433.pdf
- Ask EMA (mail: info@ema.europa.eu)
These databases could also be interesting for you
Related posts
The Iceberg Search: Navigating the Submerged Depths of Pharmaceutical Intelligence and Drug Databases
1. The Epistemology of Drug Data: Beyond the Visible Web In the pharmaceutical and healthcare industries, information is not merely a utility; it is the fundamental currency of survival, compliance, and therapeutic success. When a regulatory affairs manager, a...
Find German Equivalent of US Drugs – A Guide for Pharma Professionals
Find German equivalent of US drugs – it’s a challenge that pharmacists, healthcare providers, and pharmaceutical analysts often face. A patient arrives with a U.S.-branded prescription, or a formulary review requires checking if an American medication is available in...
OTC and Rx Drug Database Germany: The Complete Guide for Pharma Professionals
Entering Germany’s pharmaceutical market requires navigating a vast landscape of prescription (Rx) and over-the-counter (OTC) drug information. From regulatory nuances to pricing and reimbursement details, having all data at your fingertips is crucial. An OTC and Rx...
Schedule a free demo
Book your personal 30-minute demo and experience how pharmazie.com can revolutionize your daily work routine.
Personalized demo for your industry
Live access to all functions
Individual questions & answers
Non-binding & free of charge