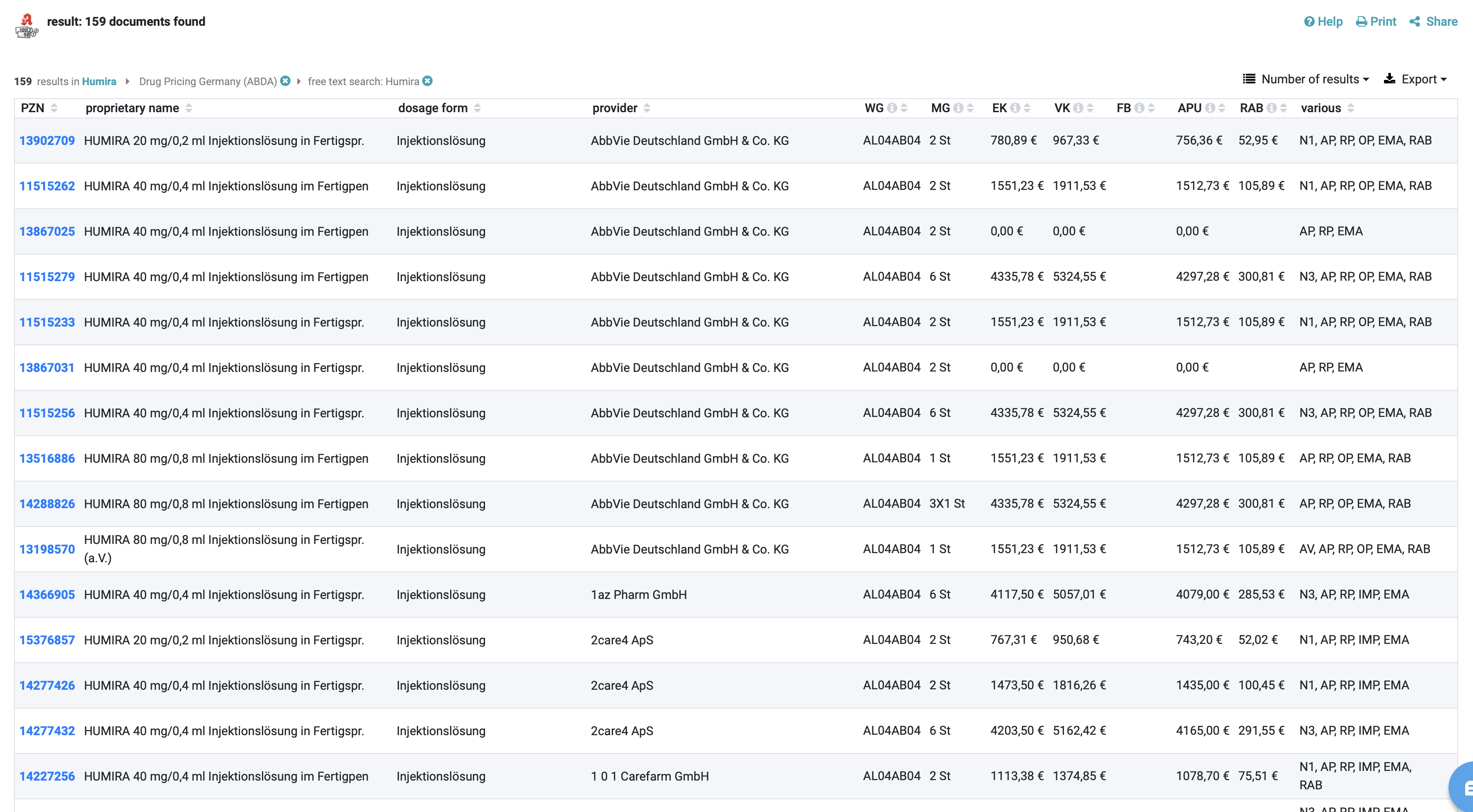
Up-to-date and reliable: The DRUG Pricing Germany Tool
The ABDA Article Master (Drug Pricing Germany Tool ) addresses key market access challenges in the German pharmaceutical industry:
- Comprehensive pricing data for complex, regulated prescription medication pricing
- Biweekly updates reflecting new drugs, rebate contracts, and regulatory changes
- Information on drug substitutions, rebate contracts, and framework agreements
- Legal details on prescription status, eligibility, and relevant laws for compliance
- Up-to-date pricing information for all stakeholders in the German market
- Centralised, reliable source of pharmaceutical data, addressing national infrastructure challenges
Key Features and Benefits
- Detailed Information: The tool includes comprehensive data on drug characteristics, pricing, and legal requirements
- Regular Updates: Biweekly updates ensure the most current information is available
- Unique Identifier: Uses the Pharmazentralnummer (PZN) for unmistakable article identification
- Price Calculation: Assists in complex price calculations for prescription drugs, considering regulations like AMPreisV
- Supplier Database: Includes an integrated address database of article suppliers
- Market Access Support
The ABDA Article Master facilitates market access by: - Providing transparency in the complex German pharmaceutical pricing system
- Ensuring compliance with frequently changing regulations
- Offering crucial data for pricing decisions and negotiations
- Supporting pharmacies, health insurance companies, and pharmaceutical companies in their daily operations
By addressing these pain points, the ABDA Article Master and the Drug Pricing Germany Tool helps manage the complexity of the German pharmaceutical market and supports market access for pharmaceutical companies
Comfort package for enterprises
Add-on (optional): PZN Individual
- upload contents for pharmaceutical central numbers (PZN)
- custom list content
- filter configuration
- data export to excel or csv
- customised pricing competitor analysis and report
- “4-preisgünstigsten” (four most cost-effective) analysis and report
Suitable for:
Pharma Industry, Pharma Trade, Health Insurance, Regulatory
Source: ABDATA PHARMA-DATA-SERVICE
Eschborn, Germany
Related subscription
This database can be accessed after purchasing the subscription package ‘Drug Pricing’ or ‘Premium’
What customers say
I am working with pharmazie.com everyday, especially with the Drug Pricing Tool Germany. Dapou Pharma is a Pharma-wholesaler & Parallel Trader and needs to have access to secure and up-to-date drug prices. The best in pharmazie.com is always to know which product is marketed in which country. Also the contact details of the pharmaceutical producers and marketing authorisation holders are most helpful.
CHECK OUT THIS DATABASE IN 5 STEPS
Background information
The most important abbreviations in the drug pricing tool Germany
The most important abbreviations in the database ABDA article master estimate:
- AP Pharmacy required
- BTM narcotic
- DRO drugs
- EK pharmacy purchase price
- IMP reimport
- KP clinic pack
- N1 Therapy-appropriate pack size N1
- N2 Therapy-appropriate pack size N2
- N3 Therapy-appropriate pack size N3
- NA non-pharmaceuticals
- NL negative list
- OR original preparation
- RP prescription
- VK Pharmacy Sales Price
A unique identifier: the Pharmazentralnummer (PZN)
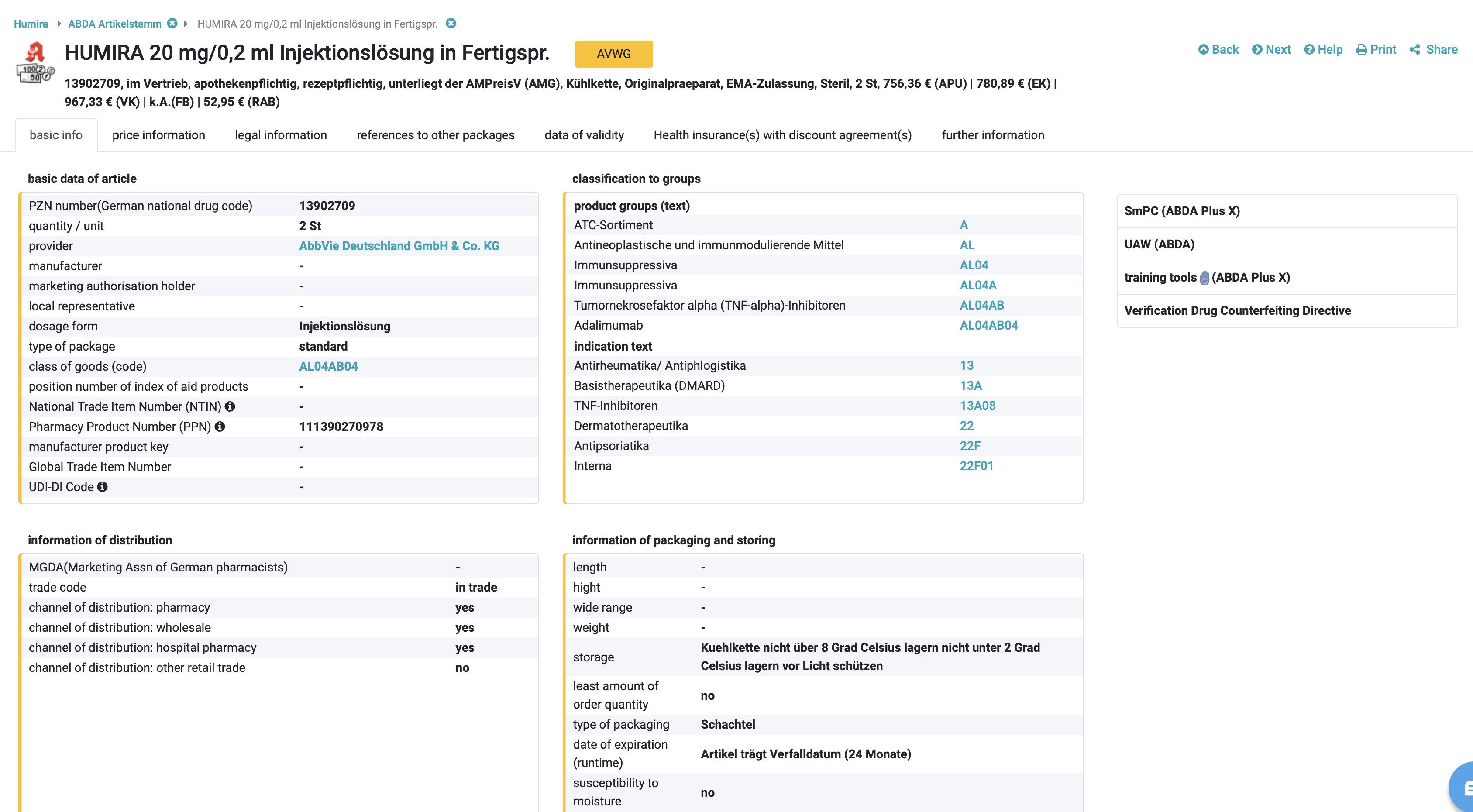
The basic data in the DRUG Pricing Database (ABDA-Artikelstamm) primarily serve for an unmistakable identification of the article. In addition to the PZN, attributes such as article description, dosage form, package size including unit of measurement of the quantity, article type and ABDATA product group code provide detailed information about the article.
The article description distinguishes between short name (26 digits) and long name (50 digits). For ranges such as compression and support stockings, the long names provide detailed information on quality, colour and size. The article type is used for differentiation if the short name, dosage form, package size and supplier are identical. This is the only way to distinguish the so-called standard packaging of a finished drug from hospital packaging, pandemic articles or approved canned goods for patient-specific drug supply. Each article is assigned an alphanumerically structured ABDATA merchandise group code. There are A and B material group codes. The A merchandise category code primarily identifies drugs requiring pharmacy services. In the A area, the character string following the first character (A) corresponds to the WHO ATC code . The ABDATA commodity group code AN02BA01 (Tab. 1.) results accordingly for Aspirin® tablets.
Example of an A commodity group code
|
A |
ATC assortment |
|
AN |
Nervous system |
|
AN02 |
Analgesics |
|
AN02B |
Other analgesics and antipyretics |
|
AN02BA |
Salicylic acid and derivatives |
|
AN02BA01 |
Acetylsalicylic acid |
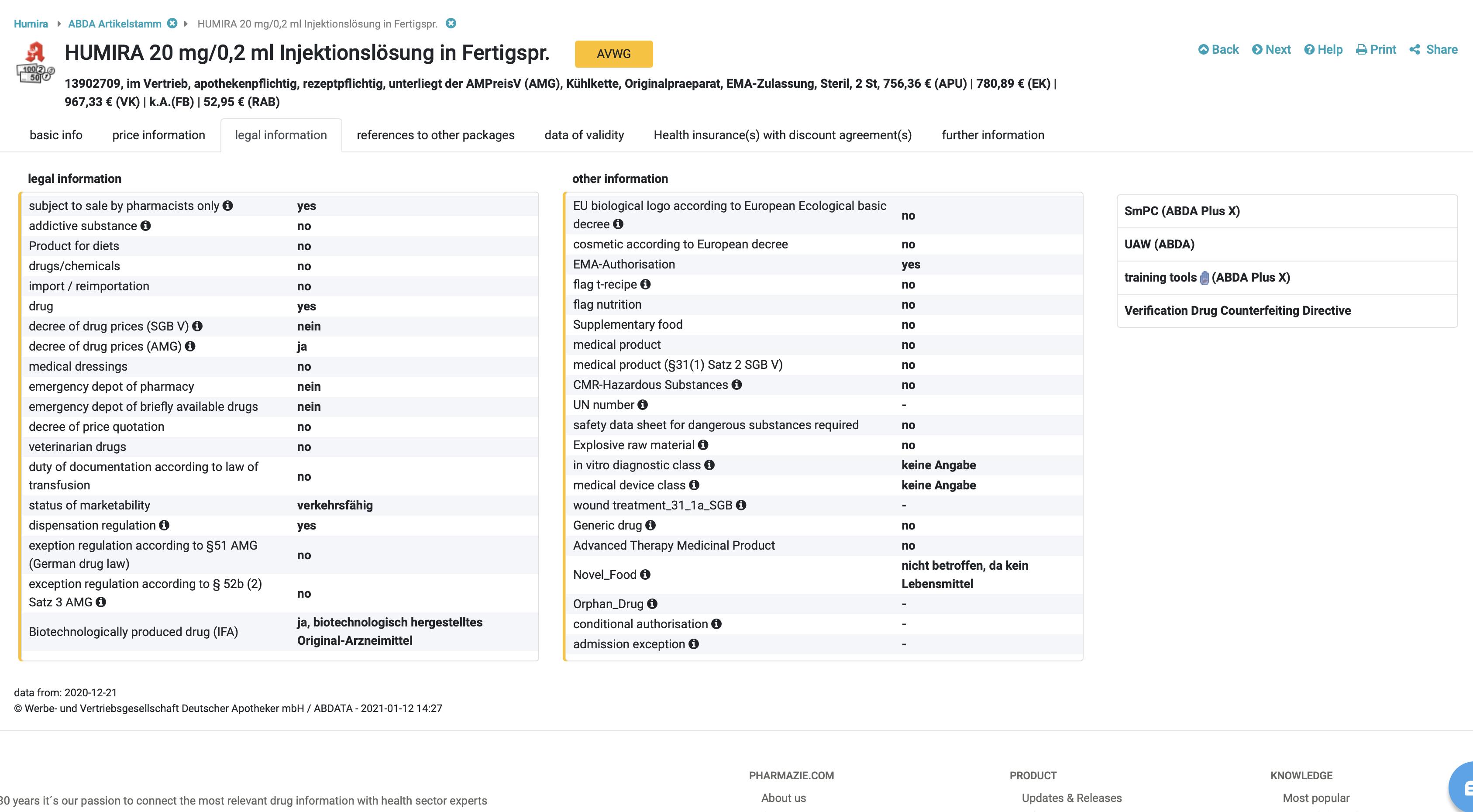
Everything that is right: The legal information
Article classification as well as the corresponding tax regulations can be found in the legal information in the DRUG Pricing Database. If articles are assigned to certain categories, the other can exclude them. For example, an article cannot be classified as both a drug and a medical device at the same time. Other indicators also apply: A veterinary drug is always also flagged as a drug. The following categories are distinguished:
- Drugs according to AMG[1]
- veterinary medicines
- Medical device according to valid medical device law
- Drug/chemical (this includes substances which are used in pharmacies as starting material is used for the manufacture of medicinal products or other products and laboratory substances).
- Dietetic according to the regulation on dietetic food, including reference to § 31 SGB V [2]
- Food supplements according to NemV[3]
- Food according to LFGB [4]
- Cosmetic according to Regulation (EC) No. 1223/2009
- authorised biocide or plant protection product
[1]AMG: Arzneimittelgesetz (German Medicine Law)
[2]Dietetic according to §31 (5) SGB V: Balanced diets for enteral nutrition reimbursable within the scope of drug supply
[3]NemV: Nahrungsergänzungsmittelverordnung (Ordinance on Food Supplements)
[4]LFGB: Lebensmittel- und Futtermittelgesetzbuch (Food and Feed Code)
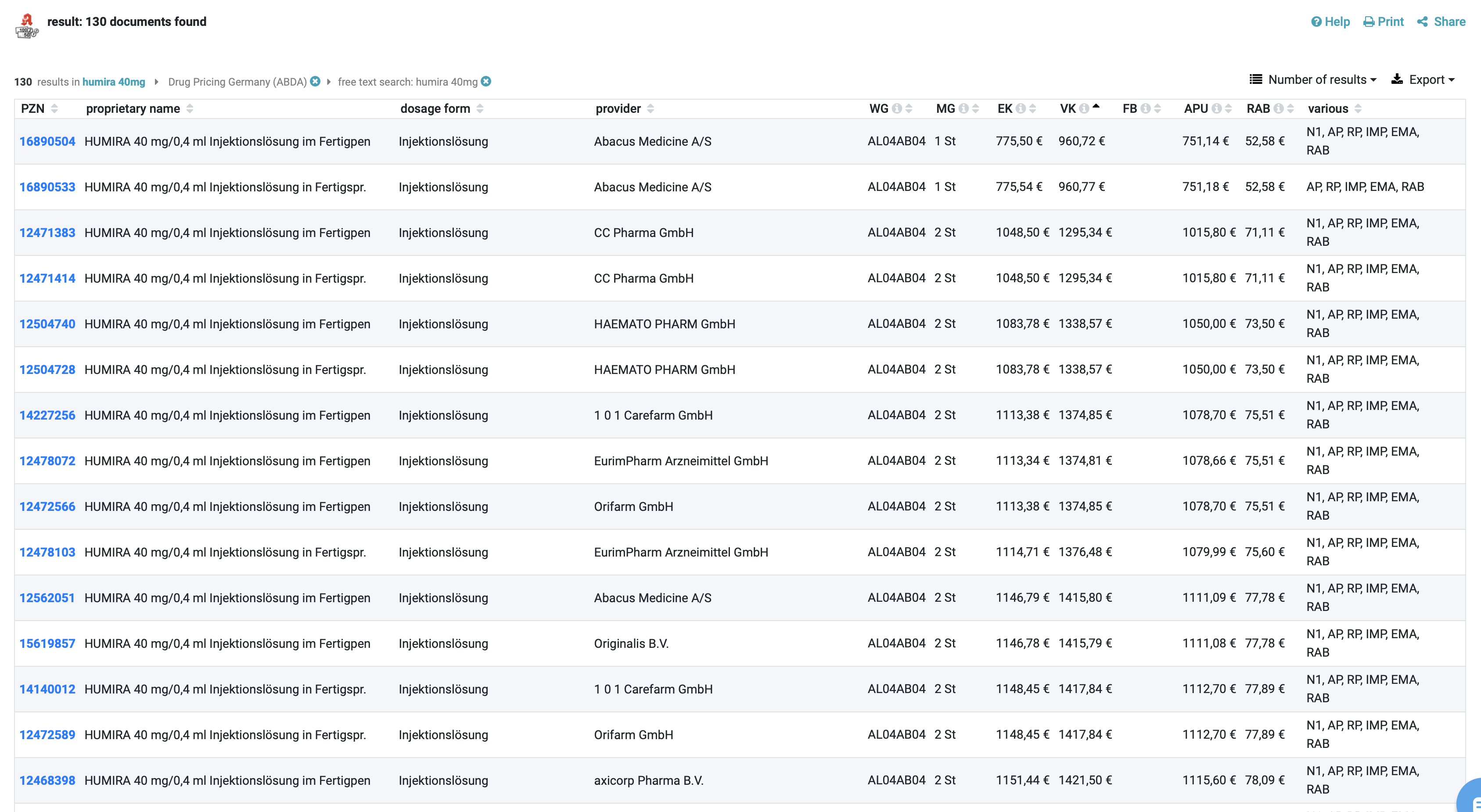
Down to the last cent: price information
Price information is at the heart of the article information within the DRUG Pricing Database (ABDA-Artikelstamm) and its additional module ABDA-Artikelstamm Plus V. For prescription drugs, pricing is complex and strictly regulated. The AMPreisV according to AMG serves to calculate these prices and regulates fixed surcharges on the manufacturer’s selling price up to the final price, which is the same in all pharmacies.
All about Distribution: Sales and Reference Information
The references to the distribution channels reported by the supplier provide important information on the purchase of the articles. A distinction is made between distribution via public pharmacies, pharmaceutical wholesalers, hospital pharmacies or pharmacies supplying hospitals or other retail outlets. If an article is a clinic module, reference is made – if reported by the supplier – to the corresponding clinic packs of which this article is a component. A hospital module describes a finished drug package that is a subunit of a hospital package and may not be dispensed separately.
The supplier informs about the sales status whether his articles are “in sales”, “out of sales” or “withdrawn”. Articles that are marked as “not for distribution” may be sold off, provided there is nothing to the contrary (such as their marketability). A reference indicator (PZN) is used to refer to a successor product for articles that are “not for sale”. The referenced follow-up article is a sensible alternative with the same or similar intended purpose; suppliers of drugs or medical devices are required to interpret their notifications so narrowly that drug/product safety is guaranteed. Only drugs which are withdrawn from the market in connection with the negotiations on the reimbursement amount (in accordance with § 130b SGB V and are therefore no longer intended for sale and distribution) are given the “withdrawn” sales status. These articles will therefore be deleted from the database three months after their identification. However, this is an extremely rare case.
An address database integrated in the Drug Pricing Database (ABDA-Artikelstamm) informs about the suppliers of the listed articles. It is based on the IFA data and is supplemented by the service lines created by ABDATA. The service lines provide information about further communication and information channels that facilitate direct contact (e.g. to the medical-scientific department).
Not above 8 °C: Packing and Storage Information
For the storage of the articles different data are essential. For example, it is necessary to know which storage temperatures must not be exceeded or undercut for certain articles. In the case of temperature-sensitive articles, the reference to transport within a cold chain must also not be neglected. Information as to whether articles should be stored in a dry place, whether there is a risk of breakage, whether they should be stored lying down or upright, or whether they should be protected from light or sunlight can be found in the Drug Pricing Database (ABDA-Artikelstamm) for specific packs.
These databases could also be interesting for you
Related posts
Pharmacy Wholesale Drug Prices in Germany: Ensuring Transparency, Access, and Compliance
Pharmacy wholesale drug prices in Germany are a critical concern for international pharmaceutical wholesalers looking to stay competitive and compliant. Germany is Europe’s largest pharmaceutical market, and its drug pricing system is highly regulated – making market...
Drug Pricing Database Germany: A One-Stop Solution for Up-to-Date Pharmaceutical Prices
Drug pricing database Germany – these four words sum up a critical need in the pharmaceutical industry. In a market as regulated and dynamic as Germany’s, professionals across market access, pharmacovigilance, hospital pharmacy, and health insurance face the constant...
Pharma Pricing Germany: Navigating Costs, Compliance and Transparency
Pharmaceutical pricing in Germany is unique in Europe – offering initial free pricing yet governed by strict mechanisms like AMNOG benefit assessments, reference pricing, and rebate contracts. In this comprehensive guide, we explain pharma pricing in Germany for...