The new packaging law 2019 – who is affected?
The new Packaging Law (VerpackG) entered into force on 1 January 2019 and replaced the Packaging Ordinance (VerpackV) that had previously been in force. The new regulations entail some important changes for manufacturers and dealers – pharmaceutical manufacturers, pharmaceutical wholesalers and pharmacies are also affected by the law. Anyone who fails to comply with the law is threatened with heavy fines of up to 200,000 euros.
Why all this?
The Packaging Law and the Zentrale Stelle Verpackungsregister (ZSVR) were introduced in Germany in order to specify product responsibility for packaging and to guarantee a transparent and fair allocation of disposal costs in the market. Everyone who places packaging on the market in Germany is responsible for its proper disposal and must take care of this before it is even sold – regardless of whether it is packaging for the protection of products, for better marketing or for shipping. Because not every company can collect its packaging from the end consumer again, the so-called Packaging Schemes (Dualen Systeme) have been established, which officially take care of recycling via the yellow and blue bins and the waste glass containers – the Schemes ensures that all packaging to be licensed with a packaging scheme is collected separately and that the recycling requirements of the Packaging Law are met. Companies are required to register their packaging quantities with a Packaging Scheme and thus assume responsibility for proper disposal. This objective was already pursued with the Packaging Ordinance (VerpackV), but the obligations were ignored by a large number of market participants – the result: free riders and higher costs for those market participants who complied with the ordinance. With the Packaging Law and the ZSVR, a central packaging register was introduced to counteract this abuse. This will ensure transparency and control in the fulfilment of product responsibility.
Who is affected by the law?
All manufacturers affected by the law are players who, for the first time in Germany, sell packaging to a third party and thus place it on the market – irrespective of whether this involves the sale, consumption or use of the packaging. These players therefore include not only producers but also importers, wholesalers, online retailers and pharmacies. Each of these initial distributors of packaging to be licensed with a packaging scheme is obliged to participate in an officially approved disposal scheme and to register with the ZSVR. All manufacturers who have registered with the Zentrale Stelle are published on the website of the ZSVR – this ensures full transparency for all market participants. The end customer can also check whether the manufacturer or retailer of the product has registered and thus see if he assumes responsibility for the disposal of his packaging.
What types of packaging are subject to the law?
In principle, all packaging to be licensed with a packaging scheme must be licensed via the scheme and the Zentrale Stelle. Any packaging that is generated as waste by the end consumer is considered to be packaging to be licensed with a packaging scheme. These include sales packaging, shipping packaging (in which the product is repackaged in order to be shipped to the end consumer – for example, cartons or filling material), service packaging (packaging that is only filled with goods by the final distributor) and outer packaging (these can be additional packaging for sales packaging – this includes, for example, additional boxes in which several products are offered together). Pharmaceutical manufacturers, pharmacies and wholesalers must therefore register as manufacturers and license the packaging material they produce or use. For pharmacies and pharmaceutical wholesalers, shipping and service packaging are particularly relevant:
Shipping package
Packaging in which medicinal products are repackaged for delivery by a courier service or by mail order at the pharmacy must be licensed by the pharmacy itself. The pharmacy itself is thus obliged to register with the ZSVR.
Service packaging
Service packaging is only filled with a product in the pharmacy itself. This includes, for example, containers or bags in which the drug is handed over to the customer. In the case of service packaging, it is possible that it is not the pharmacy but the supplier of the packaging that participates in the scheme – if this procedure is agreed, the pharmacy itself does not have to take care of registration and licensing, but must ensure and check whether the supplier is actually registered and whether the service packaging participates in the scheme. It is strongly recommended to request proof of the licensing of the packaging in order to be able to prove that the packaging is licensed when requested by the Zentrale Stelle. For example, some wholesalers take over the scheme participation for their pharmacies as customer service.
Who is responsible for licensing?
In general, the person responsible for licensing is the person who manufactures the packaging and places it on the market filled with goods as the first player – who ultimately is the manufacturer or first distributor depends not only on the type of packaging but also on the packaged goods.
- Own brands
In the case of own brands, the manufacturer himself is responsible for licensing and registering the packaging.
- Goods produced in Germany
Everyone who produces packaging in Germany and sells it filled with goods as the first player must license and register this packaging. If it is a producer of sponges who sells these sponges packed in plastic to a retailer, he must license the product packaging himself. In the case of containers, for example, which a pharmacy fills with an ointment and then sells, the pharmacy itself may be responsible for licensing, since it is the first player to sell the packaging filled with a product (although, as explained above, there is an exception here for the supplier to agree to license the packaging).
- Imported good from abroad
The person who imports the goods into the scope of the Packaging Law is regarded as the first distributor and must license and register the packaging material accordingly. For example, if goods are imported from America, the importer must license all the packaging material.
- Shipping goods
The party who has filled the shipping material with goods is responsible for this. If it is an online shop that sends its own goods packed in shipping material, it is also responsible for licensing. If the shipment is carried out by a fulfillment partner who packs the goods in shipping material, this partner is responsible for licensing.
What do pharmaceutical manufacturers, wholesalers and pharmacies have to do now?
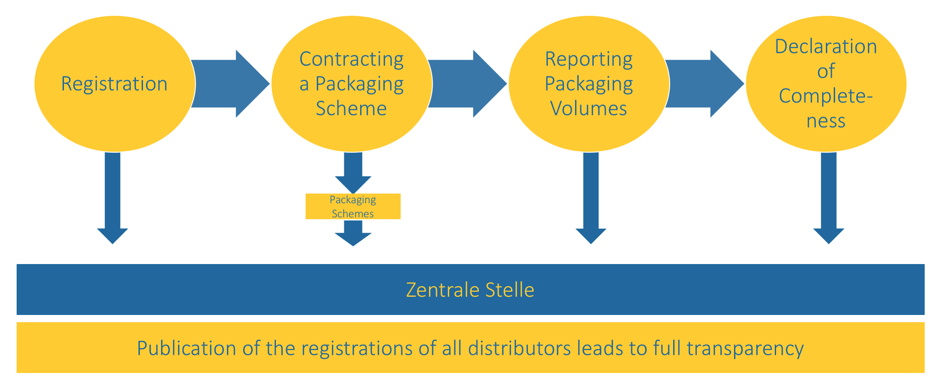
Whoever places sales packaging, service packaging, shipping packaging or outer packaging on the market for the first time must license the packaging material in order to support proper recycling. In order to guarantee this, some basic obligations apply for first-time distributors and thus also for you as a pharmaceutical manufacturer, pharmaceutical wholesaler or pharmacy.
- Before the packaging is placed on the market, the initial distributor must register in the Packaging Register of the Zentrale Stelle Verpackungsregister – this is done online via LUCID https://www.lucid.verpackungsregister.org. When registering, everyone receives their own registration number.
- The first distributor must register the packaging subject to scheme participation with a Packaging Scheme using this registration number in order to participate in the recycling scheme.
- The initial distributor must report the weight of the packaging he has placed on the market and the type of material in question once a year both to the Zentrale Stelle and to the Scheme with which he has registered its packaging (planned quantity).
- The initial distributor must provide the Zentrale Stelle with a declaration of completeness for the previous year, stating the quantity of packaging material per type of material he has placed on the market. The declaration determines what the quantity of packaging actually placed on the market is and whether more packaging has accrued than initially notified (actual quantity notification) – this only applies if a certain quantity threshold has been exceeded.
- If the first distributor imports packaging subject to Scheme participation, he must ensure that the manufacturer of the packaging is registered in LUCID. The same applies if the first distributor uses service packaging for which the supplier of the packaging has undertaken to license.
In the public database LUCID everyone can see which dealer is registered. This also enables customers to see whether the dealer acts responsibly and ensures that the packaging is properly recycled.
What penalties are there?
The following applies: A violation of the Packaging Law is an administrative offence and can be punished with fines of up to 200,000 Euros. The public register makes it easy to see who complies with the licensing obligation and thus supports fair competition – in addition to fines, there is also the threat of warnings under competition law. Wrong or even not made quantity reports can be punished with fines of up to 100,000 Euros. If one does not participate its packaging in a system one is threaten a selling prohibition – this selling prohibition affects both the manufacturer and each subsequent dealer. The public system makes it possible to see immediately which products may be sold in Germany. If goods are sold whose packaging is not properly registered, a fine of up to 100,000 Euros may be imposed.
The most important facts for pharmaceutical manufacturers, pharmaceutical wholesalers and pharmacies
As a pharmaceutical manufacturer of medicinal products which are packaged by you and placed on the market for the first time, you must register with the Zentralen Stelle and participate in a Packaging Scheme. The same applies if you, as a pharmacy, send medicines in shipping packaging (e.g. via a courier service) to the end consumer. If, as a pharmacy, you distribute medicines in service packaging such as containers, you must ensure that your suppliers are registered with the Zentralen Stelle and comply with the Scheme participation obligation.
Via the Drug Pricing Germany Tool provided by pharmazie.com, you can easily and quickly check whether your supplier is registered. Check out the Drug Pricing Germany Tool with manufacturer’s registration number. Schedule a free demo and learn more about our databases.
Related articles
IFA Pharmaceuticals and their way into pharmaceutical databases
IFA Pharmaceuticals and their way into pharmaceutical databasesHow does the IFA medicines information get from the ABDA article master to pharmaceutical databases like pharmazie.com? This question is answered in the following article. You will learn who the IFA is,...
The Planetary Health Diet
The Planetary Health Diet 37 scientists from 16 countries have gazed into the future, and it will not work without our help! You can read in this article how exactly this diet and the rescue of the planet will work. Today we are confronted with an ever-increasing...
The new Falsified Medicines Directive 2019 – which medicines are affected?
The new Falsified Medicines Directive 2019 - which medicines are affected? As of 9. February 2019, the Falsified Medicines Directive (2011/62/EU), which was adopted in 2011, has officially come into force. What does this mean for the affected players such as...