The new Falsified Medicines Directive 2019 – which medicines are affected?

As of 9. February 2019, the Falsified Medicines Directive (2011/62/EU), which was adopted in 2011, has officially come into force. What does this mean for the affected players such as wholesalers, pharmacies and pharmaceutical companies?
Background
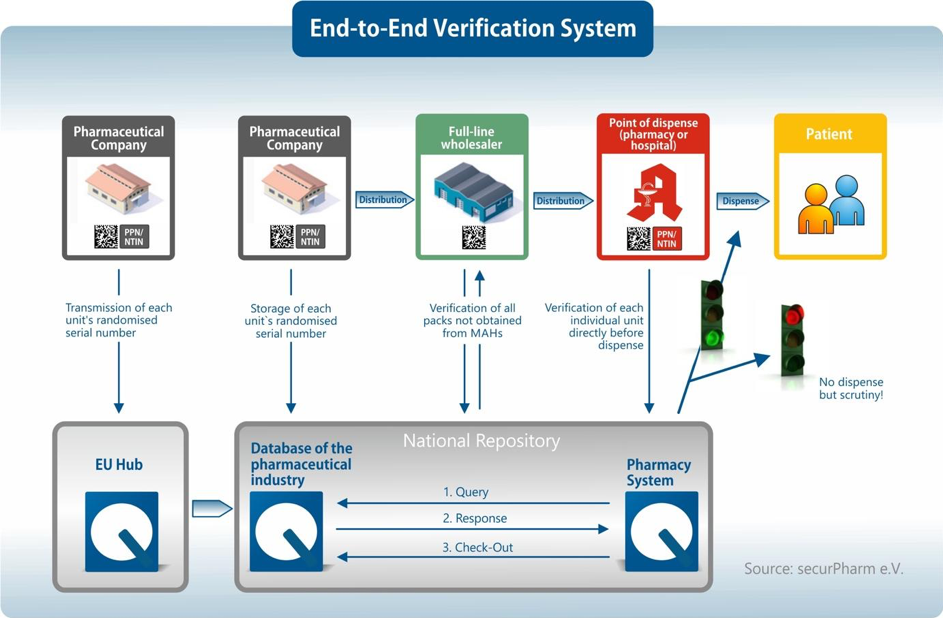
To protect against falsified medicines, which may be of low quality or wrongly dosed, an End-to-End Verification System has been introduced in the EU under the Falsified Medicines Directive. This verification system provides for the authentication of medicines, which is made possible by mandatory security features on the packaging and a comprehensive Europe-wide database system. With the help of the Falsified Medicines Directive, the distribution channel for pharmaceuticals will become even safer in the future.
From the 9th of February 2019 on, a unique identifier (UI) and anti-tampering device (ATD) is obligatory for each pharmaceutical product subject to verification in order to be placed on the market. If medicines were approved for sale before the 9th of February, they may also be sold without security features until their expiry date. However, this could lead to a drug being available on the market in three different versions: one without a safety feature, one with a safety feature but without being recorded in the database, and one with a safety feature and recorded in the database.
Which measures does the directive include?
The entry into force of the Falsified Medicines Directive has set Europe-wide measures in motion to successfully fight falsified medicines. The pharmaceutical’s appearance is also accompanied by stricter rules for the import of drug substances and documentation for wholesalers. In order to be able to determine the authenticity of the medicines, a total of two security features has been established, which must be verified before the drugs are sold.
1. Unique Identifier (UI)
Each pharmaceutical package is equipped with an individual and unique identification feature that is displayed in a two-dimensional Data Matrix Code. The Data Matrix Code includes the serial number, a product code, a reimbursement and identification number, the batch number and the expiration date. This makes each package unique and clearly identifiable by the product code. If the code on the packaging is scanned, the comparison with the database system can be used to provide feedback as to whether the Data Matrix Code actually exists and what the status of the package is. If the code cannot be found in the system or if it is noted that this package has already been sold, it may be a falsified medicine.
2. Anti-tampering decive (ATD)
In addition to the unique identifier, the medicines concerned must in future also be provided with an anti-tampering device. The anti-tampering device is used to verify whether the packaging of the drug has already been opened before. It is up to the pharmaceutical company to determine exactly what this initial anti-tampering device looks like.With the help of the security features and the End-to-End Verification System, it is possible to systematically verify whether it is a counterfeit drug, especially at the end of the supply chain in pharmacies.
Who is affected by the directive?
The Falsified Medicines Directive affects all actors in the supply chain. From the introduction of the Directive on, each of these actors, have to fulfil a particular responsibility.
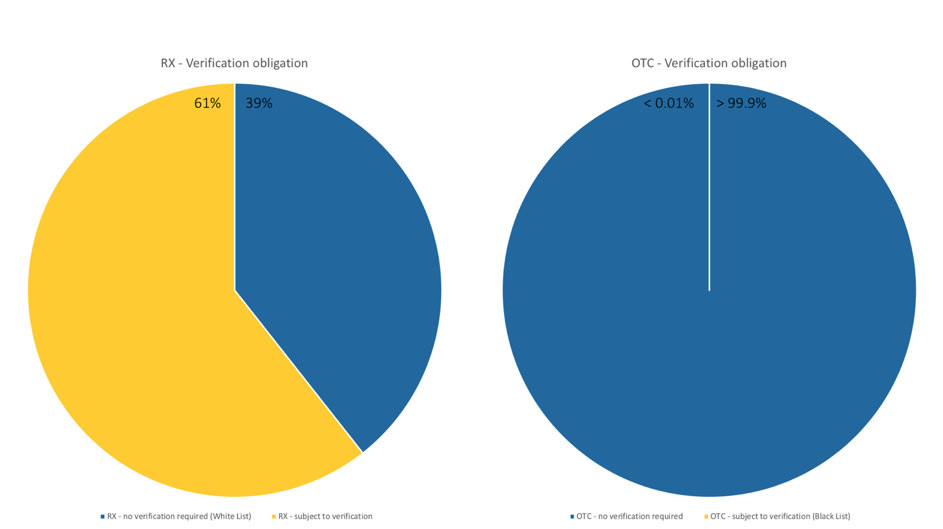
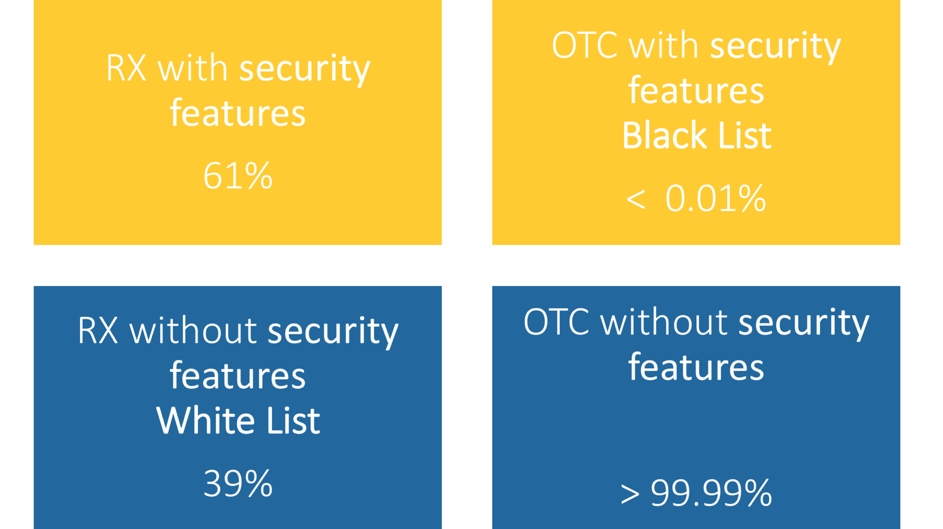
Which medicines are afftected by the EU Falsified Medicines Directive?
Which drugs in Germany are now subject to verification following the Counterfeiting Directive?
As a licensee of the Drug Pricing Germany Tool of
How can I identify a medicine that requires verification?
Important for pharmaceutical companies, pharmaceutical wholesalers, parallel traders and pharmacies: The EU member states can extend the verification obligation in the form of the unique identifier and the anti-tampering device to other medicines. The list of medicines subject to verification can therefore be continuously updated. The Drug Pricing Germany database in pharmazie.com will therefore be regularly updated with corresponding information. This includes not only the drugs concerned, but also technical details of the safety features, modalities of verification and the administration of the data system. As soon as this happens, phramazie.com will inform you about all important developments and news – so you are always up to date!
With all these new regulations and processes, it is important to maintain an overview: pharmazie.com has compiled all the important information on the new Falsified Medicines Directive for you. Here you will also find the current list of medicines affected by the Falsified Medicines Directive that require verification.
Download Excel File
with approx. 58,000 FAMs (PZN, Name, MAH, pharmaceutical dose form, verification status):
Related Articles
Navigating the Transatlantic Pharmaceutical Landscape: A Comprehensive Analysis of ABDA Standards, FDA Mutual Recognition, and Market Access in Germany (2025)
Executive Summary: The Dual Challenge of Compliance and Strategy The global pharmaceutical landscape of 2025 is defined by a paradoxical dynamic: regulatory harmonization on one hand, and increasing market complexity on the other. For pharmaceutical professionals...
The Central Nervous System of Modern Healthcare: A Comprehensive Analysis of Medication Databases, Market Access, and Clinical Safety Infrastructure
Executive Summary In the contemporary healthcare ecosystem, the medication database has transcended its traditional role as a static repository of pharmaceutical nomenclature. It has evolved into a dynamic, mission-critical infrastructure that underpins patient...
The Iceberg Search: Navigating the Submerged Depths of Pharmaceutical Intelligence and Drug Databases
1. The Epistemology of Drug Data: Beyond the Visible Web In the pharmaceutical and healthcare industries, information is not merely a utility; it is the fundamental currency of survival, compliance, and therapeutic success. When a regulatory affairs manager, a...