Drug Pricing Tool German Medicinal Products – ABDA-Artikelstamm (Taxe)

The Drug Pricing Tool German Medicinal Products – ABDA-Artikelstamm (Taxe) includes all economic data for all drugs sold in pharmacies in Germany.
Pressed to make pricing decisions? Need to negotiate the price of your drug? The Drug Pricing Tool provides prices of approved branded and generic drugs across the German market such as prices, package sizes, registered numbers for ordering, etc. The Bundesvereinigung Deutscher Apothekerverbände, abbreviated ABDA, supplies comprehensive information on release and billing of drugs common to pharmacies. In this context, complex and difficult regulations and legal texts are converted into comprehensible overview exposés that make life easier in day-to-day business.
The stock list contains all information required for the distribution and billing of medical products and other products typical to pharmacies as well as pharmaceutical legal information and other packaging related data.
„Drug Pricing Tool German Medicinal Products – ABDA-Artikelstamm (Taxe)”- update every two weeks
Provider of all these data is the IFA, Informationsstelle für Arzneispezialitäten GmbH . All the comprehensive, detailed declarations of the pharmaceutical manufacturers are collected and merged here. The ABDATA pharma-data-service checks the quality, edits the data and amends necessities to make the data available for the Artikelstamm. These researched and gathered data are updated every two weeks and provided to [pharmazie.com]. Every pharmaceutical manufacturer must order a central pharmaceutical number at the IFA (Pharmazentralnummer, abbreviated PZN) for each product and must describe it adequately regarding to characterisation.
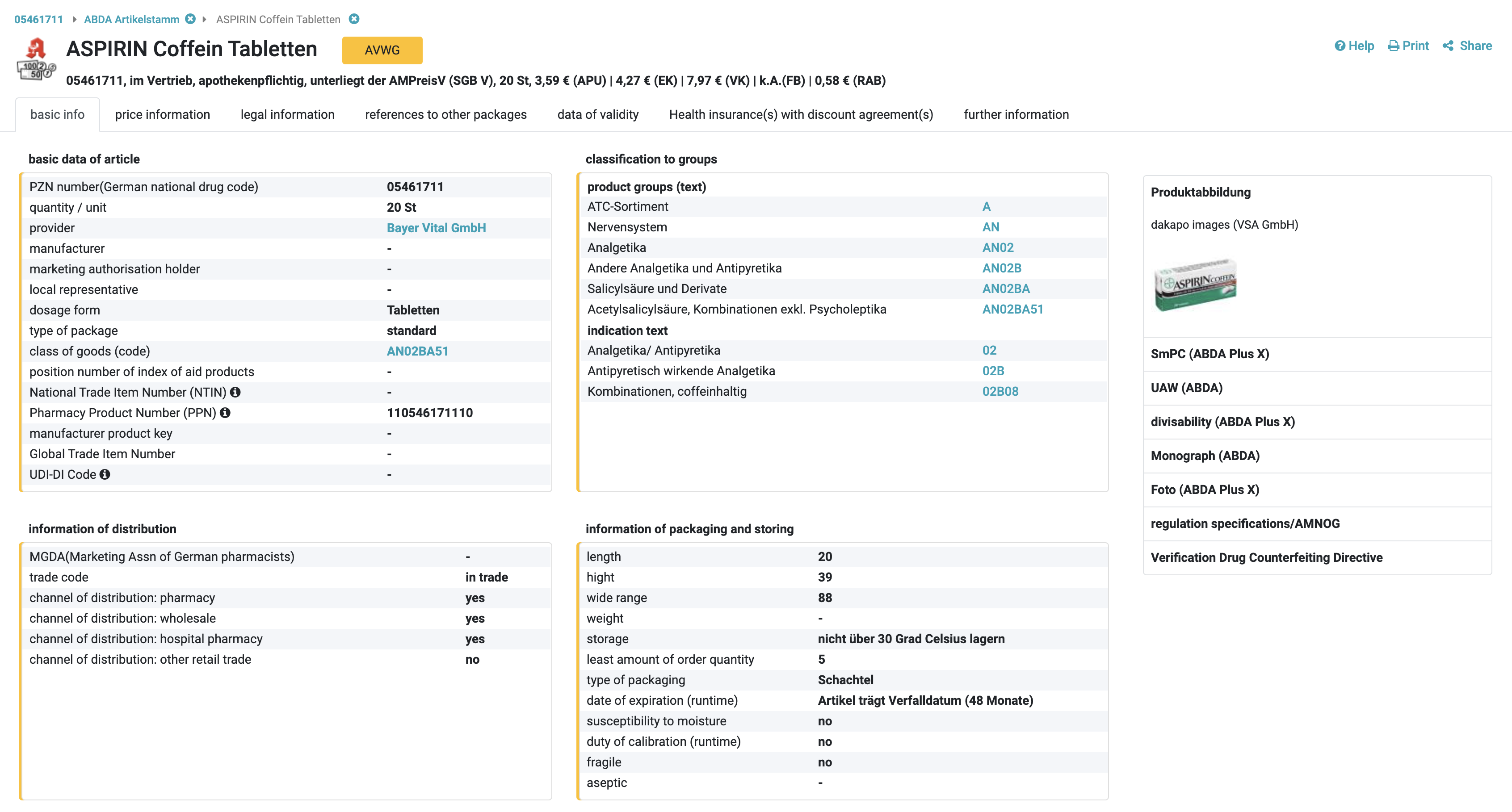
In order to state the amount of information as detailed as possible, every PZN corresponds to a great number of information attributes. In the Artikelstamm you have access to many details in
- central pharmaceutical number (PZN)
- name of article, long name of article up to 50 signs and practical sorting outcome
- package size and packing unit; identification of package sizes suitable for therapy and commerce (N-badge); substitution: alternative amounts (for example puffs)
- legal information
- dosage form
- ABDATA-Warengruppenschlüssel (ABDATA class of good key): the ABDATA-Warengruppenschlüssel is an economic key for assortment and allows analyses and research
- provider release-, purchase price for pharmacies, sales price for pharmacies, sales tax information, Festbetrags- (reference pricing- and Zuzahlungsinformationen (co-payment information); Herstellerabschläge (manufacturers rebates)
- releasing instructions (such as obligation to only sell on prescription, etc.), indication on narcotic agents law BtMG (Betäubungsmittelgesetz, abbreviated BtMG) und transfusion law (Transfusionsgesetz, abbreviated TFG) as well as t-recipe
- negative list (Negativliste); indication on appendices of directives on drugs (u. a. OTC-abstract, compendium on medical products that can be prescripted, restrictions in prescription according to amendment III, therapeutic notes according to amendment IV
- sales information in detail
- stocking advice, period of validity
- drug storage according to §15 ApBetrO (pharmacy business rules)
- suppliers with postal address, e-mail, website as well as telephone and telefax numbers of certain divisions, for example order taking, medical-scientific information (Service-Lines)
In a Nutshell:
- prices
- changes in products
- Abrechnungsmodi
- accounting modes
- legal demands
- information on packaging
- discount agreements according to § 130a Abs. 8 SGB V
Because the legislative authorities change and add new requirements regularly, [pharmazie.com] always has a lot of work to do. The PZN, for example, are labelled according to the negative list and import medication, which caused an aut-idem regulation for example with the Arzneimittelausgaben-Begrenzungsgesetz (drug release limiting law). This means, that the medical doctor can indicate “aut idem” (“or something similar”) on the prescription in order to enable the pharmacist to hand out another than the named product to the customer, but with the same active agent.
Hereby, the rules of § 130a Abs. 8 SGB V for the realization of the sales discounts agreements are met.
[pharmazie.com] also integrates information into the Artikelstamm according to the law “Beitragssatzsicherungsgesetz“ to
- sales discounts by pharmacies
- discounts by the pharmaceutical manufacturer
- discounts by wholesalers
- as well as the amount of discount in each case.
Get all important information in our Whitepaper
Related Articles
Navigating the Transatlantic Pharmaceutical Landscape: A Comprehensive Analysis of ABDA Standards, FDA Mutual Recognition, and Market Access in Germany (2025)
Executive Summary: The Dual Challenge of Compliance and Strategy The global pharmaceutical landscape of 2025 is defined by a paradoxical dynamic: regulatory harmonization on one hand, and increasing market complexity on the other. For pharmaceutical professionals...
The Strategic Imperative of the Summary of Product Characteristics (SmPC): Regulatory Frameworks, Digital Transformation, and AI-Driven Compliance
Executive Summary The global pharmaceutical ecosystem is currently navigating a period of unprecedented complexity. At the epicenter of this environment—balancing the rigorous demands of regulatory compliance, the clinical needs of healthcare professionals, and the...
Pharmacy Wholesale Drug Prices in Germany: Ensuring Transparency, Access, and Compliance
Pharmacy wholesale drug prices in Germany are a critical concern for international pharmaceutical wholesalers looking to stay competitive and compliant. Germany is Europe’s largest pharmaceutical market, and its drug pricing system is highly regulated – making market...