The AMNOG / HTA benefit risk assessment procedure in Europe and Germany
The European Regulation (EU) 2021/2282 on health technology assessment (HTAR) entered into force on 11 January 2022 and applies from 12 January 2025 on.
In Germany basically all authorised medicines entering the market are reimbursed. Before the introduction of the Arzneimittelmarktneuordnungsgesetz (AMNOG) in 2011 there was a system of free-pricing and full reimbursement in Germany.
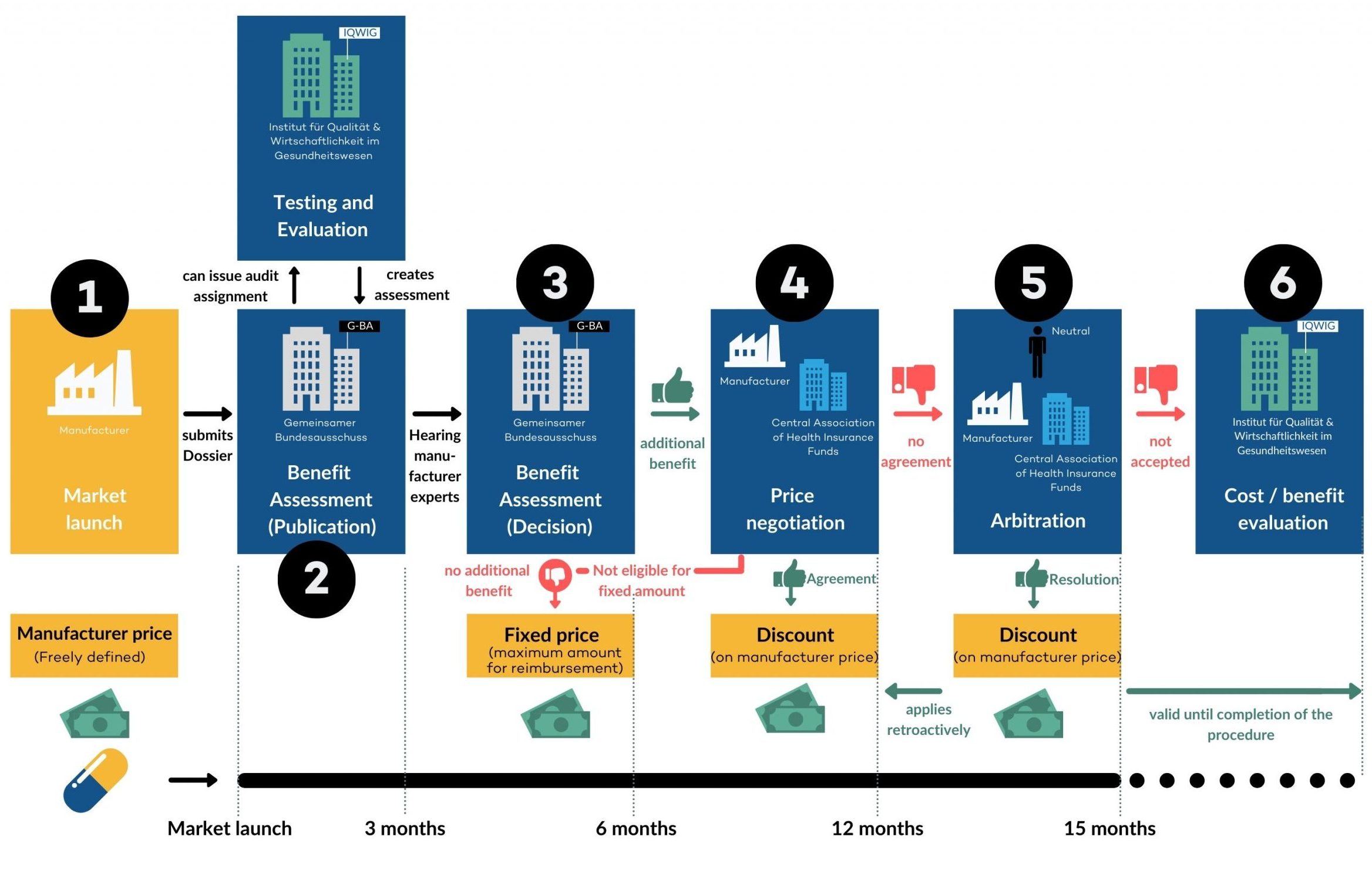
According to the German Drug Regulatory Affairs Act (AMNOG or HTA), manufacturers of pharmaceutical drugs must prove the additional benefit of a drug when it is launched on the market. The Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) determines which proof is required for the respective drug. Subsequently, the submitted evidence is reviewed by the Institute for Quality and Efficiency in Health Care (IQWiG). This process is called AMNOG benefit assessment.
If the additional benefit could be substantiated, negotiations are held together with the Association of Statutory Health Insurance Funds (GKV-Spitzenverband) to determine the amount of reimbursement that should be allocated.
If no additional benefit could be determined according to the AMNOG benefit assessment, the amount of the market price for the drug will be limited. Alternatively, the drug is grouped with comparable drugs for which a fixed amount already applies (reimbursement ceiling). If no agreement can be reached, the final decision is taken by an impartial third party.
AMNOG Database
In our AMNOG Benefit Assessment Database you can see all the necessary information on current and completed benefit risk assessment procedures at a glance:
- Dossier of the pharmaceutical entrepreneur
- Information on the comparative therapy
- Information from IQWiG’s benefit risk assessment
- Minutes of the oral hearing
- Resolution & Reasons
- AMNOG mandatory discounts
- European Comparison Price
Suitable for:
Updates: monthly
Source: G-BA (Gemeinsamer Bundesausschuss)
Berlin, Germany
Related subscription
This database can be accessed after purchasing the subscription package ‘Drug Pricing’ or ‘Premium’
Product Tour
AMNOG Database – Early Benefit Assessment (IQWIG / G-BA)
- most user friendly
- no need for translation of the data into English
- price history is simply available at one place
- price comparison and price changes are available with export to Excel
- Search prices by active ingredients or ATC
- Get your individual AMNOG Update Report on changes relevant
to you into your mailbox twice a month (PharMonitor) - Upload custom data to the AMNOG Database
- Updated monthly
The AMNOG benefit risk assessment and price evaluation in Germany
FAQ’s
What functions does the AMNOG Benefit Assessment Database include in detail?
This database compiles information on the benefit assessment of medicinal products in accordance with the Act on the Reform of the Market for Medicinal Products (AMNOG). Resolutions are partly also translated into English.
FULL-TEXT SEARCH IN AMNOG
- Simply search through the resolutions of the Federal Joint Committee (G-BA), including the reasons and the IQWiG’s concise benefit assessment
- The direct connection of the documents to the German Drug Pricing Database enables a structured database search via the pharmaceutical central number (PZN) and other criteria
ACCESS TO INFORMATION ON THE OFFICIAL GBA PROCEDURES WEBSITE:
- Profile, deadlines, title and commentary of the procedures
- Documents of the decision-making process
INFORMATION RESULTING FROM THE GBA DECISIONS
- area of application
- additional benefit
- relevant and purposeful comparative therapy
- yearly therapy costs
INFORMATION OF THE MAIN REASONS OF THE GBA:
- Reason & course of the limitation of the period of validity
- Duration of the consultation
INFORMATION FROM THE VERBATIM RECORDS OF THE GBA:
- Participants
- Duration of the hearing
INFORMATION FROM THE DATA OF THE GBA:
- Comparison: pharmaceutical companies vs. IQWiG vs. G-BA
- full text search in the summary of the GBA benefit risk assessment
SPECIAL PROCEDURES:
- Indication expansions
- Look up inventory market
- Grouping in fixed amount group
- Closed, exempted and open procedures
Save your AMNOG searches in the German Drug Pricing Database:
- Easily create and classify your own search queries
- Search directly for keywords within the ABDA Database
- Export of the results to Excel
- Display of prescription requirements
- Integration of registration documents and package inserts
- Daily news from the federal institutes
OPTIONAL MODULES:
- Price histories of AMNOG drugs on pharmacy central number basis via our PharMonitor
- Individual contents: The AMNOG Benefit Assessment Database can be expanded to include access to other databases.
For example, monitoring, price history or discount agreements with health insurance companies - Price history per PZN
What are the current challenges of the AMNOG benefit risk accessment procedure?
Over the past years, the AMNOG procedure has proven to be a reliable means of fairly pricing drugs with added value on the market. Nevertheless, the AMNOG procedure is still facing challenges.
These include:
- The requirements of the regulatory authorities must always be aligned with the design and implementation requirements of the GBA at an early stage – both nationally and at the European level
- There must be no excessive price pressure on pharmaceutical companies, which would prevent or hinder the market launch of important drugs and thus innovation
- The legally binding prescription of medicines with reimbursement must be given by the doctors. The final selection of the appropriate medication should be based purely on the needs of the patient and not on AMNOG
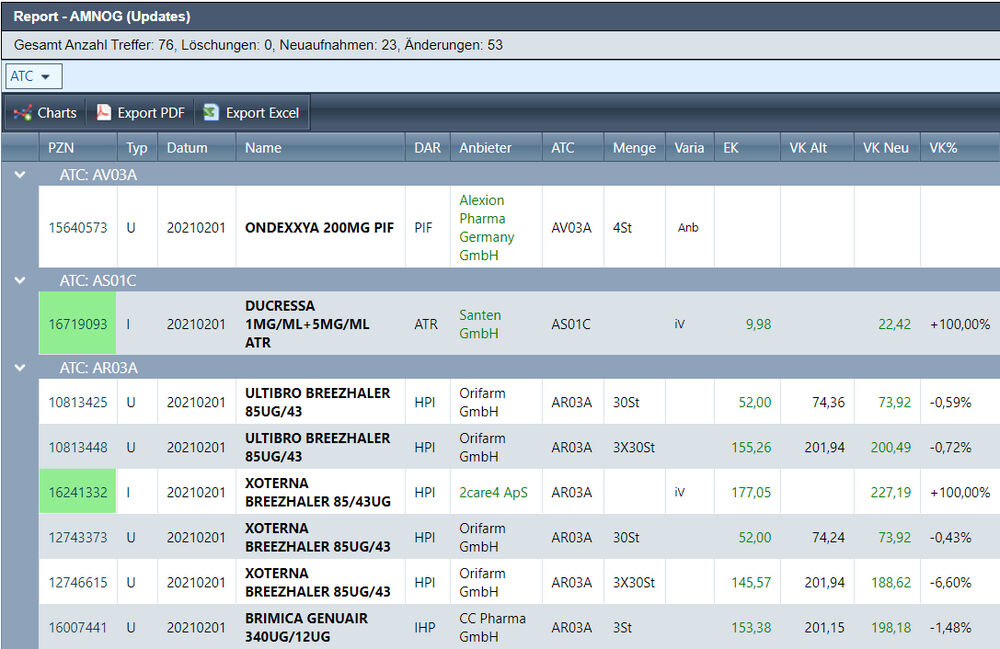
How does the AMNOG procedure work?
The AMNOG procedure is intended to provide information on whether a new drug has an additional benefit compared to the previous standard. In order to prove an additional benefit, the respective pharmaceutical entrepreneur can prepare a dossier. As soon as the new active ingredient is approved, the dossier is submitted to the Federal Joint Committee (G-BA) and then evaluated by IQWIG. After the dossier has been evaluated, the G-BA can then determine whether or not the drug has an additional benefit and thus influence the final manufacturer’s price at which the drug is offered on the market.
Source: IQWIG.de
https://www.iqwig.de/de/presse/mediathek/grafiken/das-amnog-verfahren.12439.html
These databases could also be interesting for you
Related articles
Navigating the Transatlantic Pharmaceutical Landscape: A Comprehensive Analysis of ABDA Standards, FDA Mutual Recognition, and Market Access in Germany (2025)
Executive Summary: The Dual Challenge of Compliance and Strategy The global pharmaceutical landscape of 2025 is defined by a paradoxical dynamic: regulatory harmonization on one hand, and increasing market complexity on the other. For pharmaceutical professionals...
The Strategic Imperative of the Summary of Product Characteristics (SmPC): Regulatory Frameworks, Digital Transformation, and AI-Driven Compliance
Executive Summary The global pharmaceutical ecosystem is currently navigating a period of unprecedented complexity. At the epicenter of this environment—balancing the rigorous demands of regulatory compliance, the clinical needs of healthcare professionals, and the...
Pharmacy Wholesale Drug Prices in Germany: Ensuring Transparency, Access, and Compliance
Pharmacy wholesale drug prices in Germany are a critical concern for international pharmaceutical wholesalers looking to stay competitive and compliant. Germany is Europe’s largest pharmaceutical market, and its drug pricing system is highly regulated – making market...
Schedule a free demo
Book your personal 30-minute demo and experience how pharmazie.com can revolutionize your daily work routine.
Personalized demo for your industry
Live access to all functions
Individual questions & answers
Non-binding & free of charge