Access to an accurate, up-to-date European drug pricing database is essential for healthcare professionals in Germany, Austria, and Switzerland. Whether you’re calculating reimbursement, comparing market prices, or sourcing alternatives during shortages, having reliable pricing data at your fingertips saves time and ensures better decisions. In this article, we analyze the search intent behind “European Drug Pricing Database” and show how a comprehensive solution like pharmazie.com meets the needs of pharmaceutical industry experts, wholesalers, pharmacies, hospitals, and reimbursement authorities in the DACH region. (Spoiler: with pharmazie.com’s integrated databases, you can get all the drug pricing and availability information you need – plus safety checks and AI tools – in one trusted platform.)
Why Healthcare Professionals Need a European Drug Pricing Database
In Europe’s complex pharmaceutical market, drug prices vary by country and change frequently due to regulations and reimbursement decisions. For professionals across the supply chain, this creates daily challenges. Who benefits from a centralized European drug pricing database?
- Pharmaceutical Industry: Market access and pricing teams must track pricing regulations (like Germany’s AMNOG outcomes) and reference prices across countries. A centralized database helps monitor competitor prices and reimbursement conditions in multiple markets, enabling data-driven pricing strategies.
- Wholesalers & Pharmacies: Distributors and pharmacy managers need up-to-date price lists and alerts on price changes or supply shortages to manage inventory and customer orders. Quick access to official prices and generic alternatives ensures correct billing and cost-effective purchasing.
- Hospitals: Hospital pharmacists require current pricing and availability data to source medicines, especially during shortages or when importing therapies from neighboring countries. They also benefit from linked clinical information (e.g. interactions or contraindications) to ensure patient safety alongside cost considerations.
- Health Insurers & Reimbursement Authorities: Payers and agencies set reimbursement rates by referencing prices in other European countries (a practice known as external reference pricing). They need a reliable overview of international drug price benchmarks to make informed decisions on coverage and cost containment.
Simply put, a “European Drug Pricing Database” addresses a pressing informational need – aggregating pricing data from many sources into one accessible hub. Without it, professionals might juggle dozens of national databases, websites, and PDFs, risking outdated information and inefficiencies.
The Challenge of European Drug Pricing Data
Each European country has its own pricing and reimbursement system, from Germany’s AMNOG and reference price groups to Austria’s Codex and Switzerland’s lists. Staying current is tough when information is scattered. For example, EU authorities have created a collaborative database called EURIPID containing official medicine prices from many countries. This initiative shows the importance of data sharing:
“EURIPID provides an example of a value-adding initiative for countries to voluntarily participate in data sharing that promotes price transparency.” – World Health Organization, 2022 (source)
However, access to official European price databases like EURIPID is restricted to national authorities, leaving industry stakeholders and healthcare providers without direct access. Public price lists (where available) are often published in local languages and formats, making cross-country comparisons arduous. Moreover, prices can change with little notice – new reimbursement decisions, policy updates, or supply issues can render last month’s data obsolete.
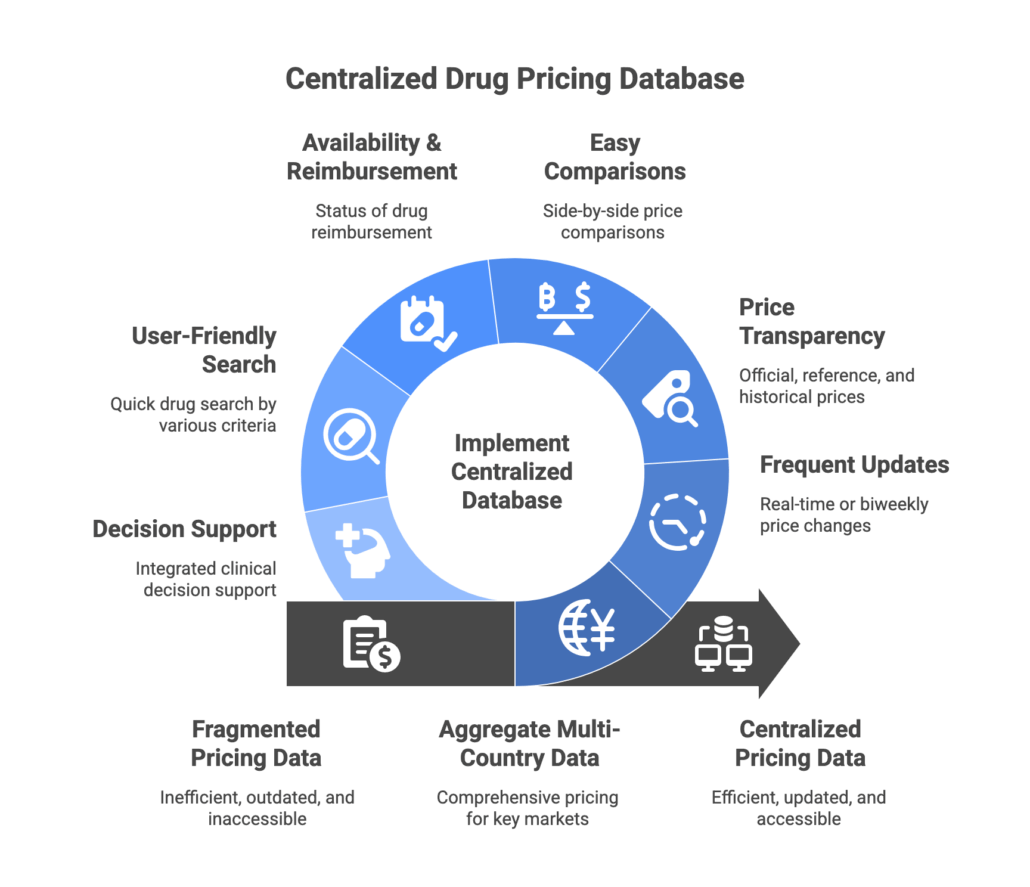
What does this mean for healthcare professionals? It underscores the need for a single, comprehensive drug pricing database that:
- Aggregates multi-country data – covering pricing information for all key European markets (all EU countries plus UK, Switzerland, etc.) in one place.
- Updates frequently – reflecting price changes, new product launches, and policy updates in real time or at least biweekly, so you’re never working with stale data.
- Provides price transparency – showing official list prices, reference prices, and even historical price trends or discounts for a complete picture. (For example, being able to see price history back to 2003 for Germany.)
- Enables easy comparisons – allowing users to compare drug prices across countries or between different products (original vs generic vs biosimilar) side by side.
- Includes availability and reimbursement status – indicating if a drug is reimbursed, under review, or currently in short supply, which is crucial for operational decisions.
- Is user-friendly and searchable – so that busy professionals can quickly find a drug by name, active ingredient, ATC code, or even a national ID (like PZN in Germany), without navigating multiple systems or languages.
- Offers decision-support – ideally integrating clinical decision support (like interaction checks or contraindication alerts) so that price info comes with context on safe usage, helping balance cost and patient safety.
- Integrates with workflows – via web services or APIs, so that the data can feed directly into pharmacy software, hospital information systems, or corporate databases for seamless updates and analysis.
These features define the ideal European drug pricing database. Now, let’s explore how pharmazie.com delivers on these needs.
Pharmazie.com – A Comprehensive European Drug Pricing Database Solution
Pharmazie.com is a leading pharmaceutical data platform that brings together over 25 trusted drug databases in one place. Launched over 30 years ago, pharmazie.com has grown into perhaps the most comprehensive European drug pricing database and medication information system available to healthcare professionals. It caters specifically to the needs of the DACH region and beyond, combining pricing data with rich clinical information. Here’s how pharmazie.com stands out:
- Integrated Drug & Substance Databases: Pharmazie.com consolidates data from national drug dictionaries and databases across 50+ countries. This includes the well-known sources for the DACH region – for example, the German ABDA database (with details on 50,000+ German medicines and 120,000 international products), the Austrian Austria-Codex, and the Swiss Pharmindex, as well as the German Gelbe Liste and Rote Liste® directories. All of these are interlinked, providing a one-stop reference for product information, active ingredients, therapeutic indications, and more. You can search by product name, ingredient, or even identifiers like the Pharmazentralnummer (PZN), and instantly retrieve harmonized data on composition, indications, and regulatory status across countries. This integration saves countless hours that would otherwise be spent consulting separate national databases.
- Price and Availability Modules: For users focused on pricing, pharmazie.com offers dedicated modules and tools that ensure you never miss a market change. The Drug Pricing Germany Tool (based on the official ABDA “Artikelstamm” price list) provides biweekly updates of all German medication prices, including prescription drug prices regulated by AMPreisV, wholesale and pharmacy selling prices, and even rebate contract information. In Austria and Switzerland, pharmazie.com integrates the latest data from national sources so you have current prices in those markets as well. There’s also a Transparency List (AVWG) module for Germany – this compares the prices of original brands, generics, and parallel imports, highlighting their effects on patient co-pays, insurance refunds, and mandatory manufacturer discounts. Such price comparison tools are invaluable for quickly identifying the most cost-effective alternatives. Additionally, pharmazie.com’s system flags supply shortages and availability alerts: if a drug is temporarily unavailable in one country, users can find alternative sourcing options (for example, an equivalent product available abroad) thanks to the platform’s international scope. (In fact, pharmazie.com’s database is used by hospital pharmacies to locate international alternatives for unavailable drugs, leveraging import provisions.)
- AMNOG Database for Germany’s HTA Outcomes: One highlight for users in Germany or dealing with German market access is the built-in AMNOG HTA Benefit Assessment Database. This module provides a one-glance overview of all ongoing and completed AMNOG procedures – including the drug’s dossier, IQWiG assessment, G-BA decisions, and negotiated reimbursement prices. It even lists the final agreed price (Erstattungsbetrag) and any European price benchmarks used. Instead of sifting through lengthy G-BA reports (in German), international teams can access all key AMNOG outcomes in English via pharmazie.com. The database is updated after each G-BA plenary meeting (typically monthly), ensuring that pharma companies and payers stay up to date on the latest reimbursement statuses. This AMNOG database is a boon for market access professionals who need to track Germany’s early benefit assessments and pricing mandates closely. (As pharmazie.com notes, there’s “no need for translation” – the platform translates and consolidates the data for you.)
- Decision-Support & Risk Checks (CAVE & Interactions): Beyond pricing data, pharmazie.com enhances medication safety through its CAVE and interaction check modules. The ABDA-Database C.A.V.E. is a clinical decision support tool integrated into the platform that checks for patient-specific risks: it flags potential contraindications, allergies, age- or gender-specific warnings, duplicate therapy, and other cautionary (Latin: “cave”) alerts. In parallel, the system offers a comprehensive drug–drug interaction check powered by the same ABDA database, which covers interactions among German drugs (including food interactions). For example, a pharmacist or physician using pharmazie.com can input a patient’s medication list and immediately see if any combinations are unsafe or if patient factors (e.g. renal impairment, which is included under CAVE) present an extra risk. This goes far beyond what a basic pricing database would do. It transforms pharmazie.com into a decision-support system – you not only compare drug prices, but also ensure therapeutic safety and compliance. Such integrated checks help healthcare providers make cost-conscious choices without compromising on patient care. (As one hospital pharmacy director noted, “the CAVE-Check… represents a considerable increase in drug safety” in daily practice.) All these safety modules are continually updated biweekly alongside the pricing data, so the information remains current with the latest pharmacovigilance findings.
- AI-Powered Chatbot Assistance: Pharmazie.com has embraced AI to further improve user experience. Their ChatSmPC is an AI-based chatbot trained on up-to-date pharmaceutical information (including Summaries of Product Characteristics for drugs). It acts like a virtual expert assistant: you can ask the chatbot complex questions about drug uses, dosages, contraindications, or regulatory details and get instant, accurate answers – even outside of normal business hours. This tool is available 24/7 and always uses the latest data, which means healthcare professionals get quick answers without manual searches. For example, a medical affairs specialist could ask, “What’s the recommended dose adjustment of Drug X in renal impairment?” and the ChatSmPC will respond based on the official SmPC – saving time digging through documents. By integrating an AI chatbot for pharmaceutical data, pharmazie.com enhances productivity and ensures that critical drug information is never more than a question away. (Notably, the AI chat is GDPR-compliant and backed by pharmazie.com’s 30 years of drug data expertise, so users can trust its responses.)
- Web Services & API Integration: Recognizing that many organizations want to embed drug data into their own IT systems, pharmazie.com offers flexible web services and API integrations. Essentially, any function you can perform on the platform – be it a price lookup, a price comparison table, or a CAVE/interaction check – can be accessed via API for integration into hospital software, pharmacy management systems, or pharma company internal tools. According to pharmazie.com, their web service enables machine-to-machine communication so that, for example, you can call up a price comparison or drug safety check directly within your own application. This is a huge advantage for enterprise users who need pharmazie.com’s data feeding into their workflows automatically. It means you can stay updated in real-time without even logging into the web interface – your systems can pull the latest prices or alerts on schedule. Moreover, pharmazie.com provides customized data export services (e.g. scheduled data downloads in CSV/Excel format) and even custom analytic reports (such as pricing competitor analyses). In short, the platform isn’t just a static database; it’s a dynamic service that can plug into your organization’s processes, enhancing efficiency and ensuring everyone is working from the same trusted data source.
All these features come with the assurance of pharmazie.com’s trustworthiness and expertise. The platform has been serving healthcare professionals for over three decades, evolving in step with regulatory changes. Pharmazie.com sources data directly from authoritative entities like ABDATA (the official source behind the ABDA database) for Germany and collaborates with many national data providers – meaning the information is as credible and official as it gets. Updates are rolled out every 14 days or faster, and even price changes can be previewed a few days in advance via the PharMonitor service (a personalized report of upcoming price changes, new product launches, and status changes in the German market). Additionally, pharmazie.com prides itself on being a neutral, independent platform focused purely on providing information – a critical factor for users who need unbiased data for decision-making.
Conclusion: Empowering Informed Decisions with Comprehensive Data
In the fast-paced pharmaceutical landscape of Europe, having a European drug pricing database you can trust is a game-changer. For professionals in Germany, Austria, Switzerland and beyond, pharmazie.com provides that single source of truth – combining integrated drug databases, up-to-date European pricing information, availability alerts, and safety tools into one easy platform. By leveraging pharmazie.com’s comprehensive data and advanced features, healthcare stakeholders can make faster, smarter decisions: pharmacists can find affordable alternatives for patients, industry analysts can craft optimal pricing strategies, and payers can benchmark prices confidently knowing they have the latest information at hand.
Pharmazie.com is more than a database – it’s a complete solution that improves efficiency (no more jumping between sources), ensures accuracy (with data updated biweekly and validated by 30 years of expertise), and enhances patient care (through decision-support modules and AI assistance). It’s no surprise that pharmazie.com has become a trusted partner for thousands of users across the DACH region, from hospital pharmacy teams to pharma company executives.
Ready to experience the benefits for yourself? Book a free demo with pharmazie.com and see how the European Drug Pricing Database and its suite of tools can be tailored to your needs. With the most comprehensive pharmaceutical data at your fingertips, you’ll save time, reduce risks, and stay ahead in the ever-changing pharma market. Contact pharmazie.com today to unlock efficient pricing research and decision support – and join the ranks of healthcare professionals who have found an easier way to stay informed and competitive.