Drug pricing database Germany – these four words sum up a critical need in the pharmaceutical industry. In a market as regulated and dynamic as Germany’s, professionals across market access, pharmacovigilance, hospital pharmacy, and health insurance face the constant challenge of tracking drug prices, reimbursement rates, and availability. An accurate and integrated drug pricing database for Germany is not just a nice-to-have—it’s essential for informed decision-making and compliance. This article explores why a unified pricing database is needed, the limitations of fragmented sources like ABDA, AMNOG and GKV databases, and how pharmazie.com provides a comprehensive solution. We’ll also highlight the benefits of a one-stop platform – from saving time to improving safety – and share expert insights on leveraging such a resource in daily practice.
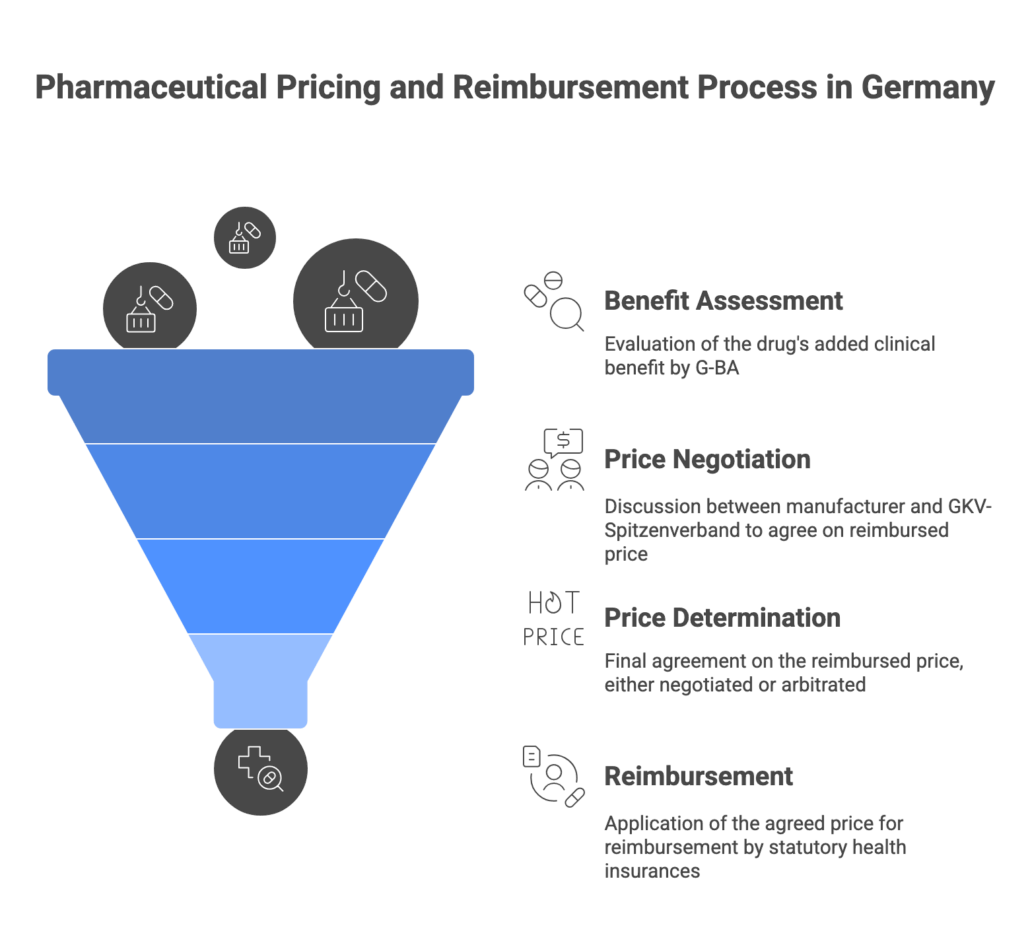
The Need for an Accurate, Up-to-Date Drug Pricing Database in Germany
Staying up-to-date with pharmaceutical pricing in Germany is notoriously complex. Prices of medications are tightly regulated and can change frequently due to new product launches, reference price updates, or negotiated reimbursement agreements. In fact, the principal industry reference (the “Lauer-Taxe(R)”, based on the ABDA database) is updated twice a month – on the 1st and 15th of every month – to reflect the latest changes. These bi-weekly updates incorporate thousands of price changes, new generics or biosimilars entering the market, and adjustments to reimbursement criteria. The National Association of Statutory Health Insurance Funds (GKV-Spitzenverband) similarly updates official reference price lists every two weeks to keep pace with market changes. For pharmaceutical professionals, this means that pricing information can become outdated within days if not monitored closely.
Why does this matter? Having accurate, real-time pricing data is critical for a variety of stakeholders:
- Market Access teams need current price points and reimbursement levels for strategy and negotiations. In Germany’s AMNOG process for new drugs, outcomes of health technology assessments and price negotiations directly impact a drug’s allowable price. Missing a recent change could mean mispricing a product or misjudging a market opportunity.
- Pharmacovigilance and Regulatory Affairs professionals must ensure that any pricing-related decisions (like patient co-pay levels or switches to cheaper alternatives) comply with the latest laws and reimbursement directives. With updates to reference pricing and discount agreements happening regularly, outdated data could lead to non-compliance.
- Hospital Pharmacy and Clinical Pharmacists rely on up-to-date prices for budgeting and therapeutic decisions. For example, if a therapy becomes available at a lower cost generic, or if a drug’s reimbursement status changes, pharmacists must know promptly to adjust purchasing and formulary decisions.
- Health Insurers (Krankenkassen) and reimbursement specialists need to verify claims against current pricing and co-pay rules. An integrated database helps ensure that reimbursements and patient charges are calculated on the correct, current prices.
In short, the search intent behind “drug pricing database Germany” is clear: professionals are seeking a reliable, unified source of truth for drug prices in Germany that is accurate, up-to-date, and easily accessible. Relying on manual checks or outdated print lists is inefficient and risky. “We need secure and up-to-date drug prices,” says Salman Lolagar, a pharmaceutical wholesaler, emphasizing that having current pricing information “in one place” is crucial for business decisions. The solution lies in integration – bringing all the relevant pricing data into a single, authoritative database that can be readily consulted or integrated into existing workflows.
Fragmented Sources of Pharmaceutical Pricing Data (and Their Limitations)
Traditionally, obtaining drug pricing information in Germany meant piecing together data from multiple fragmented sources. Each source provides part of the puzzle, but none on its own gives the full picture that modern professionals require. Let’s look at the key databases and sources for German drug prices and why using them in isolation can be problematic:
- ABDA Article Master (Lauer-Taxe(R)) – The ABDA Article Master (also known as “Lauer-Taxe(R)”) is the central pricing database used by German pharmacies. It includes every medicinal product on the market with comprehensive information required for dispensing and billing: official prices (manufacturer’s price, pharmacy purchase price, pharmacy retail price), VAT, legal categories (prescription status, narcotics status), reference (fixed) prices, patient co-payment status, and mandated manufacturer rebates. The ABDA database is extremely detailed and is considered the gold standard for pricing data – but access to it is typically through licensed pharmacy software or costly subscriptions. Moreover, it is a standalone resource; users still need to interpret how policy changes (new reference price groups, new reimbursement agreements) affect the data. Maintaining your own connection to ABDA data requires significant effort and technical integration, which not all organizations have.
- GKV-Spitzenverband Publications – The GKV, as the umbrella of statutory insurers, publishes crucial information like negotiated reimbursement amounts for new drugs (AMNOG outcomes) and reference price group updates. For example, after a new medicine undergoes the AMNOG benefit assessment and price negotiation, the agreed Erstattungsbetrag (reimbursement price) is filed and can influence the market price if it’s lower than the list price. The GKV also maintains lists of reference prices (Festbeträge) and co-payment exemptions for drugs that are sufficiently below reference pricing. However, these are often available as PDFs or web pages on the GKV or BfArM websites, separate from the day-to-day tools pharmacists use. Without integration, a market access manager might have to manually cross-check a drug’s reference price status on the BfArM site while also looking up its pharmacy price in the Lauer-Taxe – a time-consuming process prone to error.
- AMNOG Benefit Assessment Reports – Information on early benefit assessments (run by IQWiG and decided by the G-BA) and their outcomes (additional benefit granted or not, which affects pricing) is published in G-BA resolutions and databases. For each new active ingredient, one must consult G-BA documentation to see if an added benefit was recognized and what restrictions or conditions were set. If an added benefit is not proven, the drug might be subject to reference pricing or other price limits. The lack of a central, easily searchable repository for all past and current AMNOG decisions makes it challenging to quickly find the status of a given drug’s assessment and reimbursement price. Professionals often resort to consulting the G-BA website, IQWiG’s reports, or specialized newsletters to piece together this info.
- Other Drug Compendia (Rote Liste®, Gelbe Liste, etc.) – Germany has several drug compendia and databases, each with a slightly different focus. The Rote Liste® is a pharmaceutical index published by manufacturers, primarily focusing on product descriptions and Summary of Product Characteristics (SmPC) information. The Gelbe Liste is another medical drug directory which includes information on available drugs and may list prices and pack sizes, and Gelbe Liste Identa helps with identifying medications by physical characteristics. While these are useful for clinical or identification purposes, they may not always have the real-time price updates or the level of detail on reimbursement rules that the ABDA database offers. Relying on them alone could mean missing economic details (for instance, whether a drug is subject to a specific rebate contract or a new substitution rule).
- Supply Shortage Lists and Availability Notices – Recently, drug availability has become a big concern, with supply bottlenecks (Lieferengpässe) making headlines. The regulatory authority (BfArM) maintains a list of reported drug supply shortages and updates on availability. However, this is yet another separate source that professionals must monitor. A medication might be in the pricing database as available, but a recent shortage report could mean it’s actually not obtainable for some time – knowledge which is critical for hospital pharmacies and distributors. Without integration, one might not realize that a drug, though listed with a price, currently faces a supply disruption.
The problem with this fragmentation is that it forces pharmacists, insurers, and pharma companies to act like data detectives – pulling bits of information from disparate systems to answer what should be straightforward questions. Imagine trying to answer something as simple as: “What is the current reimbursable price of Drug X, and are there any special conditions (like patient co-pay waivers or restrictions)?” To get the full answer, you might need to consult the Lauer-Taxe for the list price and pharmacy price, the GKV database or AMNOG report for any negotiated rebate price, and the BfArM list for whether it’s below reference price (hence co-pay exempt). This takes precious time and opens the door to mistakes if any source is overlooked or not updated.
Industry experts note that this lack of a unified data source is a pain point. Prof. Dr. Georg Kojda, from the Institute for Pharmacology and Clinical Pharmacology in Düsseldorf, observed that traditionally “there is no other complete directory of all drugs approved in Germany” in one place. Key pricing data may be scattered across multiple outlets, each with their own update schedules. Without integration, even something routine like preparing a budget impact analysis or conducting a price comparison for therapy alternatives can turn into a manual, error-prone task.
Pharmazie.com – Integrating ABDA, AMNOG, GKV and More into One Platform
In response to these challenges, pharmazie.com has emerged as a one-stop, integrated drug information platform – effectively the comprehensive drug pricing database Germany has been looking for. Pharmazie.com brings together over 25 different pharmaceutical databases and modules under a single digital roof, providing a unified source for pricing, clinical, and regulatory information. By aggregating data from all major German sources and keeping it updated in real-time, pharmazie.com solves the fragmentation problem and offers a host of additional benefits.
Comprehensive Data Coverage from All Major Sources
Pharmazie.com’s database covers the full spectrum of drug pricing and availability data in Germany, including:
- ABDA Article Master (official price list) – At its core, pharmazie.com is powered by the official ABDA database, ensuring that users have access to the exact same pricing information used in every German pharmacy. This includes manufacturer’s list prices, pharmacy purchase prices, pharmacy retail prices, plus all the associated details like package sizes (including N1/N2/N3 pack size designations), legal status, and tax information. All economic data needed for distribution and billing are present. Crucially, this data is updated bi-weekly in sync with the national schedule, so the platform always reflects the latest price changes, new product entries, or changes in status. For example, if a new generic is added or a price is reduced on the 1st of the month, pharmazie.com will have that updated by the time it takes effect. Each product is indexed by its Pharmazentralnummer (PZN), ensuring precise identification.
- Pricing Modifiers and Reimbursement Details – Pharmazie.com doesn’t stop at listing the prices; it also integrates the contextual information that affects actual reimbursement. This includes reference prices (Festbeträge) for drugs in reference price groups, and flags when a product’s price lies above or below those reference thresholds. Co-payment information is included – for instance, if a drug is 30% under the reference price and thus eligible for patient co-pay exemption per GKV policy, that status is noted. Likewise, the legally mandated manufacturer rebates (Herstellerabschläge) under SGB V §130a are incorporated, so users know the net effective cost to insurers. All of this aligns with the data provided by IFA/ABDATA and the GKV-Spitzenverband, meaning you can see at a glance the “true” price context (not just the list price) for each drug. According to one industry blog, the Lauer-Taxe (and by extension pharmazie.com’s data) shows not only list prices but also “mandatory rebates, reference group price or negotiated reimbursement price… and information on discount agreements” in one place. This breadth of detail is exactly what pharmazie.com delivers in an accessible format.
- AMNOG Database (Benefit Assessments & Outcomes) – Unique to pharmazie.com is an integrated AMNOG HTA database that consolidates all the essential information from Germany’s early benefit assessment process. Users can quickly look up any new drug that has gone through AMNOG (since 2011) and find: the G-BA’s decision on additional benefit, IQWiG’s assessment summary, the GKV-Spitzenverband negotiated reimbursement price (Erstattungsbetrag) if applicable, and even the European price comparisons that were considered. Having this in one database is invaluable for market access and health insurance professionals. Rather than combing through G-BA meeting minutes or multiple PDF reports, pharmazie.com presents a complete dossier view for each drug’s market access outcome. The platform updates this module after each G-BA plenary session (typically monthly), so it stays current with the latest resolutions. For example, if the G-BA just decided on a new oncology drug’s added benefit and the price deal was reached, that information – including any mandated price discounts or special conditions – will be integrated and searchable. This AMNOG module ensures you never miss a development on new therapies entering the market.
- Transparency List (AVWG) – Pharmazie.com also includes the Transparency List as introduced by the AVWG (Arzneimittelversorgungs-Wirtschaftlichkeitsgesetz) reforms. This module provides price comparisons between equivalent products – typically comparing original brand drugs, generic versions, and parallel imports for the same active ingredient. It considers the “bonus-malus” rules from the AVWG, showing how various factors affect the net cost: such as insurer refunds, patient co-payments, manufacturer discounts, and pharmacy discounts. Essentially, the Transparency List gives a concise economic overview for therapeutic clusters, highlighting opportunities for cost savings (e.g. by substituting a high-cost original with a cheaper generic or import). Pharmazie.com updates this every two weeks in line with pricing changes, ensuring the comparisons are up to date. This is particularly useful for health insurers and pharmacy benefit managers evaluating which product to dispense or reimburse – all the data is in one table rather than scattered across sources.
- Drug Availability and Supply Status – To address the growing concern of drug shortages, pharmazie.com integrates data on drug availability. Users can see if a product is currently reported as in short supply or if distribution is halted. By pulling information from regulatory notifications and manufacturer reports, the platform can flag supply bottlenecks alongside the pricing info. For instance, if a certain vaccine or analgesic is temporarily unavailable nationwide, that status can be indicated so that pharmacies or hospitals are alerted to seek alternatives. This integration means you won’t accidentally plan on a product just because it’s listed with a price – you’ll know if it’s actually obtainable.
- International Data Integration – For those involved in parallel trade or international market analysis, pharmazie.com also links data on which products are marketed in which countries. As noted by a parallel trade expert, one of the “best [features] in pharmazie.com is always to know which product is marketed in which country,” alongside having the contact details of manufacturers readily available, all in one system. This extended scope is helpful for wholesalers and global market access teams who monitor price differences across borders.
In summary, pharmazie.com integrates all major German drug price databases – the national pharmacy price database (ABDA Article Master), the GKV’s reimbursement and reference price info, AMNOG outcomes, and more – into a single coherent platform. It effectively mirrors and enhances the content of the primary sources. As a result, users of pharmazie.com can trust that they are getting comprehensive and authoritative data. Prof. Kojda’s observation is now addressed: with pharmazie.com, there is a complete directory of all drugs in Germany, coupled with an unparalleled breadth of pricing and regulatory detail.
Key Features Designed for Usability and Integration
Having vast data is one thing; making it usable is another. Pharmazie.com is designed with features that ensure this rich pricing data is not only accessible but can be seamlessly integrated into the user’s workflow:
- User-Friendly Search and Interface: The platform allows intuitive searching by product name, active ingredient, or PZN. Complex queries – for example, searching by ATC code or therapeutic area – are supported, helping users find all relevant products and their prices quickly. Results are presented in clear tables and can be filtered or sorted (e.g., by price, supplier, etc.). For instance, a quick search for a drug like “Humira” shows all available package variants, their prices, legal status, and any notes on reimbursement in one view, as demonstrated in pharmazie.com’s own tutorials. This means even a newcomer can navigate the database with ease, without needing special training on the intricacies of German pricing.
- Bi-Weekly Updates & Alerts: As noted, pharmazie.com updates its pricing data every two weeks in lockstep with official data releases. But beyond passive updates, the platform offers notification services. The PharMonitor Germany tool, for example, is an automated alert system that sends out a personalized report of all relevant price changes 3–5 days before they take effect. This means if you’re subscribed to certain products or categories, you’ll learn about upcoming price changes or new market entries even before the official date (1st or 15th of the month) – giving you a valuable head-start. As PharMonitor is based on the same drug pricing database, it ensures no change goes unnoticed. “You receive the price changes 3-5 days before the actual deadline… and can thus still react in time,” the PharMonitor description notes. This kind of alert can be a game-changer for pharmacies adjusting their stocks or for insurance funds updating their reimbursement systems.
- Integration via API and Data Export: Recognizing that many organizations have their own IT systems (hospital information systems, ERP software, etc.), pharmazie.com provides options to integrate the data directly. Clients can connect via a secure API to pull pricing and product information into their internal systems in real time, or schedule regular data exports. Custom data exports (e.g. Excel or CSV files) can be configured to include just the fields and products you need, simplifying analyses or uploads to other databases. This flexibility means you can use pharmazie.com as a backend data service as well as a frontend tool. For example, a hospital pharmacy chain can integrate pharmazie.com data so that their purchasing software automatically references the latest prices and availability when placing orders – no manual lookup needed. In one testimonial, a large hospital-supplying pharmacy noted that they “integrated pharmazie.com into our ordering and drug information system” to ensure all their staff across 20+ hospitals have consistent, up-to-date information at hand. Integration options ensure that pharmazie.com fits into your workflow, rather than forcing you to change how you work.
- AI Chatbots for Fast Information (ChatSmPC & ChatPIL): Beyond raw data, pharmazie.com leverages artificial intelligence to help users quickly get answers from the wealth of textual information available (such as Summaries of Product Characteristics and Patient Information Leaflets). The ChatSmPC and ChatPIL are AI-powered assistants that allow users to ask natural language questions about a drug’s profile – for example, “What is the recommended dosage of Drug X in elderly patients?” or “What are the contraindications of Drug Y?” – and receive an instant answer sourced from the official product documents. This saves time combing through lengthy PDF files. For market access or medical affairs professionals, this means even complex info (mechanism of action, special warnings, etc.) is just a quick chat query away on the same platform where you’re reviewing prices. It’s like having a smart assistant by your side that has read all the SmPCs for you.
- Clinical Decision Support – Interaction and Risk Checks: A standout feature of pharmazie.com’s suite is its clinical decision support modules, particularly the drug–drug interaction checker and the C.A.V.E. (Computer Aided Verification of Risks) module. While these go beyond pricing, they are integral to the platform’s value as a one-stop solution. The interaction check (based on the ABDA database) allows users to screen for any potential interactions between medications, a critical function for pharmacists reviewing therapy regimens. The C.A.V.E. module adds another layer by checking patient-specific risks – for example, alerts for age-related dose precautions, contraindications due to comorbidities, or allergies. When dispensing a less expensive alternative medication, for instance, the pharmacist can immediately verify that the substitution is clinically sound and safe for the patient. Users in hospitals have praised this feature; as Dr. Klaus Ruberg reported, having these risk checks “always up-to-date in one simple place” alongside pricing and product info “represents a considerable increase in drug safety” in daily practice. Integrating safety checks with pricing data means that cost decisions can be made confidently without compromising on care quality.
In essence, pharmazie.com not only centralizes data but also equips users with tools to act on that data efficiently and safely. Whether it’s through automated alerts, easy exports, or on-the-fly AI assistance, the platform is built to streamline workflows for pharma professionals.
Benefits of a Unified Drug Pricing Platform for Healthcare Stakeholders
Consolidating all these capabilities into one platform yields concrete benefits for professionals in the pharmaceutical industry. Below are some of the key advantages that using an integrated solution like pharmazie.com’s drug pricing database for Germany can offer:
1. Save Time with Unified Access
Perhaps the most immediate benefit is significant time savings. Instead of juggling multiple databases, websites, and spreadsheets, users get one login, one interface for all their drug data needs. Need to check the price history of a medicine, its current reimbursement status, and whether it’s under a supply restriction? All of that can be done in one query on pharmazie.com, in a matter of seconds. This unified access is especially valuable for roles that regularly query drug data: for example, a Market Access manager preparing a pricing dossier can pull all necessary info (prices, comparators, reference groups, etc.) from one source instead of contacting different departments or external sources. The workflow efficiency gained allows teams to redirect time to analysis and decision-making rather than data gathering. In a fast-moving regulatory environment, this agility is a competitive edge.
2. Improve Decision Quality and Safety
With comprehensive data at your fingertips, decision-making becomes both faster and safer. A unified database ensures that no critical piece of information is missing when you make a decision. For instance, a hospital P&T (Pharmacy & Therapeutics) committee evaluating a formulary addition can see not just the cost of a new drug, but also if it was deemed to have an additional benefit by the G-BA and what its reimbursement price is, and check for any special prescribing restrictions – all before making a choice. This 360° view prevents costly mistakes such as choosing a therapy that might seem cheaper but is actually not reimbursed for a certain patient group, or overlooking a mandatory manufacturer rebate that affects budgeting. Additionally, built-in interaction and risk checks (the C.A.V.E. module) mean that pharmacists and clinicians are automatically alerted to safety issues while they review medication options. As one pharmacy director noted, the integrated risk and interaction checking in pharmazie.com made it easy to handle complex medication regimens “despite the complexity,” greatly enhancing medication safety in settings like oncology and geriatrics. In short, a unified platform supports better clinical and economic decisions, marrying the two aspects seamlessly.
3. Ensure Compliance and Up-To-Date Pricing
German pharmaceutical pricing is governed by many regulations – from the Arzneimittelpreisverordnung (drug price ordinance) that sets pharmacy markups, to social law rules on rebates and patient charges. Keeping decisions compliant means always referencing the latest official data and rules. By using a database that is updated in real-time with changes from ABDA, GKV, and G-BA, organizations can ensure compliance effortlessly. Pharmazie.com, for example, updates the data every two weeks in line with official sources, and even monthly for AMNOG outcomes. This means that if a new law or decision changes a drug’s status on Monday, by Tuesday your team is working with the new information. No lag, no outdated pricing in your system. Health insurers can trust that their claims processing is using correct reimbursement amounts, avoiding billing errors. Pharmaceutical companies can remain confident that their pricing strategies use the current regulatory landscape (e.g., knowing if their product has moved into a reference price group or if competitors dropped their prices). This up-to-date compliance extends to patient-facing aspects as well: pharmacies using integrated data can immediately charge the correct co-pay (or waive it) as per the latest GKV co-pay exemption list, keeping patients’ costs accurate and lawful. Overall, the risk of non-compliance due to outdated information is virtually eliminated.
4. Increase Workflow Efficiency and Collaboration
A unified drug pricing database also fosters efficiency across departments and even between organizations. When everyone is referring to the same single source of truth, consistency improves. Teams can collaborate more easily – for example, a pricing analyst can share a link or report from pharmazie.com with a colleague in medical affairs, who can trust that data without needing to double-check elsewhere. Pharmazie.com’s ability to export data or integrate into other software means that different functions (marketing, regulatory, clinical) are all tapping into the same updated data stream. This reduces duplicated effort and errors. Moreover, features like customizable filters and reports (as offered in the PharMonitor and PZN Individual add-on) allow each user to tailor the system to their needs, which boosts productivity. A health insurance specialist might set up filters to alert them only on changes to high-cost medications or orphan drugs, whereas a pharma trade (wholesaler) user might focus on new product launches and parallel import opportunities. Each gets the relevant info without noise, in a timely manner. This kind of targeted intelligence was difficult to achieve when data was siloed in different places. With an integrated platform, it becomes routine.
5. Empowered Negotiations and Market Strategy
For those in pharma companies, having all pricing and reimbursement data in one place strengthens negotiation positions and market strategies. When entering price negotiations or preparing for a launch, companies can quickly analyze the landscape: How have similar drugs been priced? What reimbursement concessions were made in past AMNOG deals? What are the current reference price levels for the therapeutic category? Pharmazie.com equips teams with hard data to answer these questions on the fly. It even allows price history tracking – so one can see how a drug’s price evolved over time, including any reductions after generic entry or changes due to policy (pharmazie.com provides historical prices and even highlights the differences via export). This insight supports more informed pricing decisions and faster preparation of value dossiers. Additionally, if you operate in multiple European markets, knowing which products are available in each country (and at what price) via the integrated international data can highlight parallel trade dynamics or reference pricing impacts. In essence, knowledge is power: by centralizing knowledge, pharmazie.com empowers companies and payers alike to make data-driven decisions that align with the latest market realities.
The benefits above ultimately translate to better patient outcomes and economic efficiency in the healthcare system. When pharmacists can swiftly choose a cost-effective alternative without risking a therapeutic mistake, or when insurers can optimize reimbursement without administrative delays, the whole system works more smoothly. A comprehensive drug pricing database like pharmazie.com becomes an enabling infrastructure for these improvements.
Conclusion: Embracing a One-Stop Drug Pricing Database for Germany
In the complex world of German pharmaceutical pricing, information is the lifeblood of good decisions. Embracing an integrated drug pricing database in Germany is no longer just an IT upgrade – it’s a strategic imperative for staying compliant, competitive, and efficient. By unifying data from ABDA, AMNOG, GKV-Spitzenverband and more, pharmazie.com exemplifies the power of a one-stop solution. It provides professionals with the confidence that no matter what pricing question arises, the answer can be found quickly and reliably on a single platform.
For market access and industry experts, this means fewer surprises and more control over pricing strategy. For pharmacists and healthcare providers, it means the ability to make patient-centric decisions that also respect the latest economic guidelines. And for health insurers and regulators, it means greater transparency and the potential for cost savings by identifying optimal therapeutic alternatives. In the words of one expert user, having “all relevant information always up-to-date in one simple place” is a game-changer – it turns data from a bottleneck into a catalyst for action.
Pharmazie.com has positioned itself as Germany’s trusted one-stop drug pricing database, combining exhaustive data coverage with intelligent tools like AI chatbots and risk checks. The result is a platform that not only answers your questions but also anticipates your needs, whether it’s alerting you to a price change or guiding you through a complex reimbursement scenario. As the pharmaceutical landscape continues to evolve – with new pricing regulations, emerging therapies, and ongoing supply chain challenges – having a partner like pharmazie.com ensures you’ll stay ahead of the curve.
Ready to enhance your pharmaceutical data workflow? By integrating a solution like pharmazie.com into daily operations, professionals can save time, reduce errors, and focus on what truly matters: delivering value and safety in healthcare. Don’t let fragmented data slow you down. It’s time to harness the efficiency of a unified database and experience the peace of mind that comes with always having the latest information at your fingertips.
Book a free demo of pharmazie.com today and see how a comprehensive drug pricing database for Germany can transform your work – from accelerating market analyses to safeguarding patient care. Embrace the future of pharmaceutical information management and ensure that you and your team never miss a beat in the fast-paced world of drug pricing and market access.