Entering Germany’s pharmaceutical market requires navigating a vast landscape of prescription (Rx) and over-the-counter (OTC) drug information. From regulatory nuances to pricing and reimbursement details, having all data at your fingertips is crucial. An OTC and Rx drug database for Germany can be your single source of truth in this complex environment. In this guide, you will learn why a comprehensive drug database is essential, what features to look for, and how pharmazie.com provides an all-in-one solution with real-time data on over 50,000 German and 120,000 international drug products. We’ll also explore use cases for key stakeholders – and how you can leverage such a database to accelerate market entry and ensure compliance.
Why You Need a Unified OTC and Rx Drug Database in Germany
Germany’s pharmaceutical information is highly regulated but fragmented across multiple sources. Important data – from official drug listings to price updates and reimbursement status – is traditionally scattered. As Prof. Dr. Georg Kojda (Institute for Pharmacology, Düsseldorf) observed, “there is no other complete directory of all drugs approved in Germany” available in one place. This means international pharma professionals often struggle to get a full overview of the German market. Prescription-only medications (Rx) and OTC products are listed in various databases (e.g., the ABDA database, Rote Liste®, GKV lists) each with separate access. Without integration, even answering a simple question – Is a given drug available, what is its current price, and are there reimbursement restrictions? – can require consulting multiple sources.
Such fragmentation is inefficient and risky. Data may be outdated or inconsistent between sources, leading to compliance errors. For example, missing a price update or a change in a drug’s reimbursement status could result in incorrect pricing or insurance claims. “We need secure and up-to-date drug prices… in one place,” emphasizes Salman Lolagar, a pharmaceutical wholesaler, highlighting how crucial centralized information is for business decisions. Clearly, a unified OTC and Rx drug database for Germany is essential for timely, accurate information and confident decision-making.
Challenges of Fragmented Drug Information (and the Solution)
Consider the range of information you must track in Germany: drug identifiers (Pharmazentralnummer, PZN), formulary status, prescription requirement, pricing tiers, reference (fixed) prices, patient co-pay exemptions, parallel trade availability, and more. Each piece comes from a different source – ABDA (for official master data and pharmacy pricing), GKV publications (for reimbursement amounts and reference price groups), AMNOG dossiers (for new drug benefit assessments), and so on. Manually collating these is time-consuming and error-prone. Industry experts note this lack of a unified data source is a pain point.
The solution lies in consolidation. A trustworthy drug database should integrate all relevant data into one platform. This means combining the complete list of German drugs (both Rx and OTC) with every detail you might need: indications, dosages, safety information, and economic data like prices and reimbursement terms. It should be continuously updated to reflect new product launches, price changes, and regulatory decisions in real time. In short, pharma stakeholders need a one-stop reference that acts as the “single source of truth” for the German market.
pharmazie.com – Your One-Stop OTC and Rx Drug Database for Germany
pharmazie.com has emerged as the comprehensive solution, integrating over 25 different pharmaceutical databases and modules under one digital roof. It is effectively the unified OTC and Rx drug database Germany has been looking for. By aggregating data from all major sources – and keeping it updated in real-time – pharmazie.com offers a single authoritative platform that covers pricing, clinical, and regulatory information.
What does this mean in practice? With pharmazie.com, you gain instant access to the official ABDA database (the gold-standard used in every German pharmacy) and much more. The platform includes:
- Comprehensive drug listings: Details on more than 50,000 German pharmaceutical products and 120,000 international products, covering both Rx and OTC medications. This includes everything from common OTC medicines to specialized prescription drugs. Each entry is indexed by its PZN for precise identification.
- Integrated data from 25+ sources: Key modules like the ABDA Article Master (Lauer-Taxe® prices), Rote Liste® monographs, German drug approvals and news, international drug directory, and even nutritional supplement info are bundled together. You no longer need to separately query multiple systems – a single search spans all relevant databases at once.
- Real-time updates: Data is synchronized with official updates on the national schedule. For example, pharmacy pricing (ABDA data) is refreshed bi-weekly in line with Germany’s updates, so new generics or price changes are reflected immediately. Reimbursement and regulatory info (e.g. AMNOG decisions) are updated right after each Federal Joint Committee (G-BA) meeting. You’ll always be working with current information.
- Deep drug information: Beyond basic facts, pharmazie.com provides in-depth knowledge: full SmPC (Fachinformation) details, indications, contraindications, dosing, side effects and even special notes (e.g. whether a tablet can be split or if it’s tube-feed compatible, which is critical in hospitals). All of this is available in a structured, searchable format. As one hospital pharmacist noted, having “all relevant information such as specialist information, divisibility and tube compatibility always up-to-date in one simple place” greatly improves efficiency in clinical practice.
- Pricing and reimbursement context: The database doesn’t stop at listing drug prices – it also shows the context behind those prices. You can see manufacturer’s ex-factory price, pharmacy retail price, and wholesale price for each product, along with any applicable reference (fixed) price groups and patient co-pay status. If a drug’s price is below the reference price (making it co-pay exempt), that is flagged. Mandatory manufacturer rebates (per §130a SGB V) are noted, and even any negotiated reimbursement prices from AMNOG outcomes are included when applicable. According to one industry blog, the Lauer-Taxe and pharmazie.com data show “mandatory rebates, reference group price or negotiated reimbursement price… and information on discount agreements” all in one place – exactly the breadth of detail needed for market access decisions.
- AMNOG & HTA data: Unique to pharmazie.com is an integrated AMNOG database that collates all the outcomes of Germany’s early benefit assessments since 2011. You can easily find the G-BA’s benefit assessment result for a new drug, see the IQWiG evaluation, and note if an additional benefit was recognized. If so, the negotiated reimbursement price (Erstattungsbetrag) with the GKV is shown; if not, you’ll see if the product falls under reference pricing or other restrictions. This saves immense time for regulatory and health economics teams – no need to hunt through multiple G-BA PDFs or databases. Everything is presented in a single dossier view, updated after each G-BA meeting.
- Transparency in cost comparisons: Pharmazie.com includes the Transparency List (AVWG) module, a powerful tool for comparing drug prices within the same therapeutic group. It lists original brands alongside generics and parallel imports, showing how each option’s cost is affected by insurance refunds, patient co-pays, and pharmacy or manufacturer discounts. Updated every two weeks, this helps identify cost-saving opportunities (e.g. substituting a high-cost brand with a cheaper equivalent) – particularly valuable for insurers and pharmacy benefit managers evaluating formularies.
- Drug interaction and safety checks: Patient safety is paramount. The integrated ABDA drug interactions module checks interactions among German medicines (including OTC products and even food interactions). Additionally, the C.A.V.E. (Critical Analysis of Patient Data for Efficacy) tool assesses a patient’s entire medication profile against risk factors to flag any contraindications or cautions. This kind of advanced interaction checking is a rare offering – as Prof. Kojda noted, “there is also no other program as good and reliable as the CAVE module for testing interactions”. Having it built into the database enables pharmacists and physicians to make informed decisions quickly.
- Global reach and parallel trade info: Because pharmazie.com links 25+ international databases, you can find out which products are marketed in which countries at a glance. This is extremely useful for parallel importers and global market analysts. For example, a medicine unavailable in Germany might be located in another country’s market – pharmazie.com will show that alongside the contact details of the foreign manufacturer or supplier. One expert in parallel trade highlighted that “the best
- in pharmazie.com is always to know which product is marketed in which country”, with all relevant contacts in one place. This global perspective is invaluable for sourcing alternatives during drug shortages or for competitive intelligence across borders.
- AI-powered tools: Staying true to innovation, pharmazie.com offers AI tools like ChatSmPC® and ChatPIL – essentially intelligent chatbots trained on the drug database. These allow users to ask questions in natural language and get immediate answers sourced from official drug information. For instance, instead of reading through a lengthy SmPC, you could ask, “What are the contraindications of Drug X?” and the AI will respond with the relevant section. The AI chat is available 24/7 and always uses the latest data, providing reliable answers quickly. This can dramatically improve productivity and knowledge access, especially for customer support or medical information teams. Notably, these AI features are built with data privacy in mind (hosted in Germany and GDPR-compliant), and can even be integrated via API or custom-trained on your own documents.
- User-friendly search and interface: Despite its complexity under the hood, pharmazie.com’s interface is designed for simplicity. You can search by product name, active ingredient, ATC code, or PZN, and the Iceberg Search (Eisbergsuche®) will sift through all databases simultaneously. Results are displayed in clear, structured tables with filters (e.g., you can sort by price or limit results to certain countries). This means whether you’re looking up a drug’s German SPC, checking if an alternative exists abroad, or simply verifying a price, you get quick answers without needing multiple tools.
In summary, pharmazie.com provides a truly comprehensive OTC and Rx drug database for Germany, consolidating every critical piece of information into one platform. It essentially mirrors and enhances all primary sources – from ABDA to GKV to AMNOG – so that you can trust the data is complete and authoritative. By offering this breadth and keeping it continually up-to-date, pharmazie.com has become a trusted resource for companies and institutions across the pharmaceutical sector.
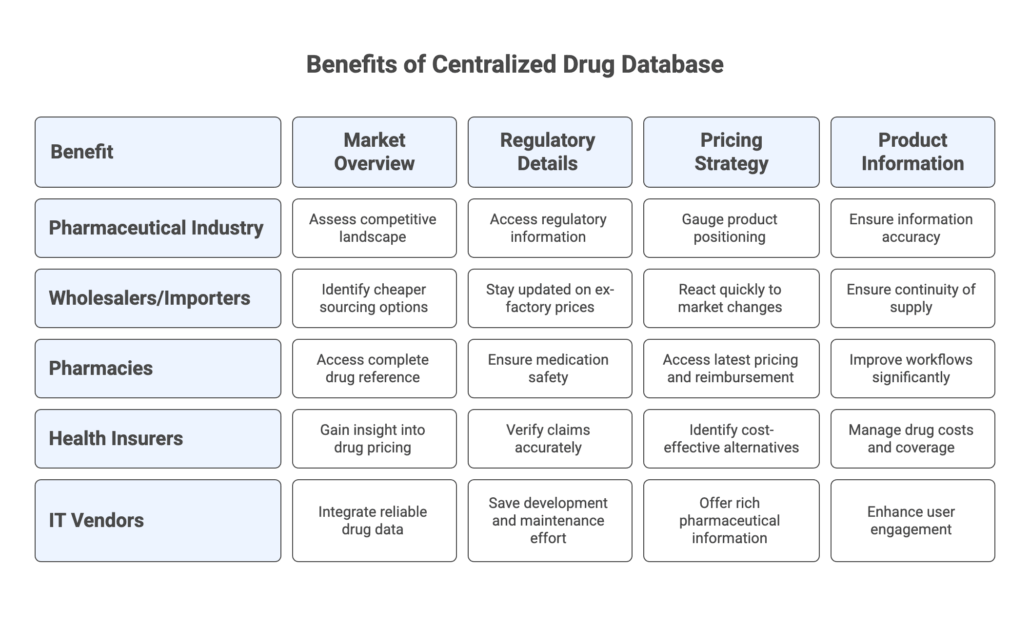
Key Benefits for Different Pharma Stakeholders
A centralized drug database like pharmazie.com delivers benefits across the entire pharma value chain. Let’s explore how various stakeholders use this tool to their advantage:
Pharmaceutical Industry (Regulatory Affairs & Market Access)
If you are a manufacturer or market access manager, you need a holistic view of the German market. Pharmazie.com allows you to quickly assess the competitive landscape: you can find all existing products (Rx and OTC) in your therapeutic area, including generics and foreign versions. This helps in portfolio planning and benchmarking. All the regulatory details are at your disposal – whether a drug is prescription-only or pharmacy-only, its ATC classification, formulations, and if any special restrictions apply.
Crucially, pharmazie.com shows you what healthcare professionals see about your products. For instance, your drug’s listing as it appears in the ABDA database or Rote Liste can be reviewed, so you can ensure information is accurate and complete. If your product goes through an AMNOG benefit assessment, you can easily retrieve the outcome and see how it’s positioned for reimbursement. There’s no need for translation of German data into English – pharmazie.com’s interface and many data fields are provided in English, which is a huge advantage for international teams.
Market access professionals also use the platform for price strategy: by reviewing the price history and current prices of analogous drugs, you can gauge where to position your product. Since discount contracts (rabattverträge) and reference prices are visible, you’ll know if your competitor has a secret discount with insurers or if they dropped below a reference price to gain co-pay exemption. All these insights enable better decision-making in pricing and reimbursement negotiations.
Lastly, regulatory affairs teams benefit from having one reference for all official drug info – from EMA authorizations (via linked EU data) to national specifics. Preparing dossiers, responding to authorities, or updating SmPC/PIL translations becomes easier when you can quickly find any required detail in the database. In essence, pharmazie.com helps industry professionals build strong product portfolios on solid data.
Pharmaceutical Wholesalers and Parallel Importers
For pharma trade companies – wholesalers, parallel distributors, exporters/importers – real-time pricing and availability data is vital. Pharmazie.com keeps you updated on current ex-factory prices (ApU) for Rx and OTC products, including any recent price changes or temporary price cuts. The platform’s price comparison and history features let you see if a product’s price has changed over time, helping in forecasting and purchasing decisions.
Moreover, knowing which products are on the market in each country can open new business opportunities. If a medicine is cheaper in another EU country, a parallel importer can identify that via pharmazie.com’s international database and consider sourcing it. The included manufacturer and marketing authorization holder contact database provides direct contact information, so you can quickly reach out to potential suppliers or partners – an efficiency boost for business development.
Wholesalers often must ensure continuity of supply. With pharmazie.com, you can check if a drug is reported in short supply or if any drug shortage notifications have been issued, allowing proactive management. Dr. Marcel Rossmy, head of pharma supply chain at Critarion AG, notes that by using the German and international databases in pharmazie.com, they could offer pharmacies and hospitals alternative sourcing options for drugs that are unavailable locally (e.g. arranging individual imports under §73(3) AMG). This kind of rapid identification of equivalent products abroad can be a lifesaver in shortage situations and is only possible when you have a broad, integrated view of the global market.
In summary, wholesalers and parallel traders rely on pharmazie.com for up-to-date prices, market intelligence across borders, and easy access to supplier contacts. It enables them to react quickly to market changes, ensure competitive pricing, and find products wherever they are available.
Hospital and Retail Pharmacies
Pharmacists in hospitals and retail settings need fast, reliable drug information at the point of care. Pharmazie.com equips pharmacies with a complete drug reference library that covers all German OTC and Rx products, plus alternatives worldwide. For a busy pharmacist, this means one login gives them the equivalent of numerous reference books and databases: the official drug directory, interactions checker, formulation details, and even patient counseling information.
Medication safety is a top priority: with the built-in interaction check (ABDA Interaction database) and the advanced C.A.V.E. risk checker, pharmacists can instantly screen a patient’s regimen for any dangerous interactions or contraindications. Instead of juggling multiple tools, a pharmacist can perform these checks within the same platform used to look up drug monographs or dosing. This not only saves time but also reduces the risk of oversight, thereby improving patient safety and care quality.
Hospital pharmacists, in particular, handle complex cases and need detailed info. Pharmazie.com includes data like whether a tablet can be crushed for feeding tubes, stability after opening, and compounding information – details often needed in a clinical setting. As one German hospital pharmacy head shared, after integrating pharmazie.com into their system, their staff across 20+ hospitals were “impressed to have all relevant information… always up-to-date in one simple place” which improved their workflows significantly.
For community pharmacies, having the latest pricing and reimbursement info at hand is crucial for dispensing. Pharmazie.com’s direct feed from the ABDA Lauer-Taxe ensures that the pharmacy knows the exact retail price, any patient co-pay, and whether a cheaper generic is mandated for substitution. The included Transparency List helps pharmacists advise on cost-effective alternatives, improving customer trust and potentially saving patients money. And because the database updates in real time, pharmacists can be confident the advice they give (or the copay they charge) is based on the latest rules – no surprises with an outdated price.
In short, pharmazie.com empowers pharmacies with comprehensive drug knowledge and decision support, enhancing their ability to serve patients efficiently and safely. It’s like having an entire pharmaceutical library and expert assistant at the pharmacy counter or ward station.
Health Insurers and Payers (Krankenkassen & Beihilfestellen)
For health insurance companies and payer organizations, controlling costs while ensuring patients get the therapies they need is a delicate balance. An up-to-date drug database is an invaluable tool for these stakeholders as well. Pharmazie.com provides payers with immediate insight into drug pricing, reimbursement status, and cost-effective alternatives.
One major use case is claims verification: insurers must check that pharmacy claims match the official prices and rules. Health insurers need to verify claims against current pricing and co-pay rules, and having an integrated database ensures reimbursements and patient charges are calculated correctly. Instead of cross-referencing claims against multiple documents (e.g., the pharmacy claim, the official price list, the co-pay exemption list), an insurer can use pharmazie.com to see all relevant info in one screen. This reduces errors in reimbursements and speeds up claims processing.
Pharmazie.com’s data on reference (fixed) prices and reimbursement amounts is particularly useful for payers. When evaluating a new drug, insurers can see if a negotiated AMNOG reimbursement price exists (post-benefit assessment) and how it compares to the list price. The system flags drugs that are “above reference price” (meaning patients would owe a co-pay) versus those “30% below reference price” (eligible for co-pay exemption). This enables insurance pharmacists to quickly decide if a drug will incur extra costs for patients or if it’s within the reimbursable range.
Moreover, the Transparency List AVWG helps payers identify where they might encourage cheaper alternatives. Since this tool shows the net cost differences between an original and its generics or imports (taking into account all the discounts and refunds), insurance formulary managers can pinpoint which products provide the same health outcome at lower cost. For example, if Drug A and Drug B are equivalents but Drug B is significantly cheaper after rebates, the insurer might promote Drug B preferentially. Pharmazie.com keeps this comparison current (bi-weekly updates), so payers always have the latest market snapshot.
Lastly, Beihilfestellen (aid offices for civil servant healthcare) and private insurers benefit similarly – they need to ensure the medications they cover are priced correctly and any applicable rules (like needing a certain generic or requiring prior authorization for an expensive new therapy) are followed. Having all German formulary and pricing rules in one database simplifies their workflows and enhances policy compliance.
In essence, pharmazie.com equips payers with the data to manage drug costs and coverage decisions with confidence, ensuring that they reimburse based on accurate, up-to-date information and can steer utilization toward value-optimizing choices.
Healthcare IT and Software Vendors
Integrating a robust drug database into healthcare software is often a challenge for IT vendors. Whether it’s pharmacy management systems, hospital EMRs, or medication dispensing apps, developers need reliable data on drugs – and they need it delivered in a flexible way. Pharmazie.com addresses this by offering API access and data integration solutions. Clients can connect via a secure API to pull the latest drug data (prices, product info, etc.) directly into their own systems in real time. This means your pharmacy software or hospital information system can automatically reference the newest information (e.g., updating a drug’s price or showing a newly launched medicine) without manual updates.
For example, a hospital software provider can use the API to ensure that whenever a doctor in their system prescribes a medication, the decision support module instantly checks the dose against the pharmazie.com drug information, or flags an interaction via the C.A.V.E. data – all behind the scenes. The user interface can remain your own, but the content is powered by pharmazie.com’s databases. This is exactly what one large hospital pharmacy network did: they “integrated pharmazie.com into our ordering and drug information system” so that staff across all departments had consistent, up-to-date data without leaving their internal application.
For software vendors targeting the German market, using pharmazie.com as a backend saves significant development and maintenance effort. You don’t need to separately license the ABDA database and multiple others, then build update pipelines – pharmazie.com has done the heavy lifting. With 30+ years of experience in drug data, the platform ensures quality and accuracy. You can retrieve custom data exports (CSV/Excel) or on-the-fly queries via RESTful API, focusing on just the fields you need (e.g., perhaps you only need price fields and ATC codes for a particular app).
Additionally, the AI chatbot features can be white-labeled via API into consumer-facing healthcare apps – for instance, a telemedicine app could integrate ChatPIL to let patients ask questions about their medications in multiple languages, enhancing user engagement with minimal effort on the developer’s part. All of this is backed by pharmazie.com’s reliable infrastructure and kept current automatically.
In summary, software vendors benefit from pharmazie.com as a plug-and-play drug data solution, enabling them to offer rich pharmaceutical information in their products quickly and cost-effectively. It ensures their end-users (pharmacists, doctors, patients, etc.) always get accurate data, while the vendors can focus on their application’s core functionality rather than data upkeep.
Conclusion: Empowering You with Germany’s Ultimate Drug Database
Navigating Germany’s pharma market becomes vastly easier when you have a powerful ally like a comprehensive OTC and Rx drug database at your side. Whether you’re managing regulatory submissions, comparing drug prices, checking interactions at the bedside, or processing insurance claims, having all the data in one reliable place saves time, reduces errors, and boosts confidence. As we’ve seen, pharmazie.com’s OTC and Rx drug database for Germany offers exactly that – an expert-curated, continuously updated, one-stop resource covering 50,000+ German and 120,000+ international drug entries with rich details from A (active ingredients) to Z (Zulassung status).
For international pharma professionals, this means you can enter and operate in the German market with a clear roadmap. No more piecing together info from disparate sources or worrying about missing a crucial update. Pharmazie.com delivers comprehensive coverage (from clinical monographs to pricing and reimbursement data) along with innovative tools like AI assistants and seamless API integration. It’s a solution built on decades of expertise, designed to meet the high standards of Germany’s healthcare system and the needs of modern pharmaceutical business.
High value and trust are at the core of pharmazie.com’s offering – exactly what you need to succeed in a regulated market. Ready to leverage the ultimate German drug database for your organization? Experience it for yourself and stay ahead of the curve. We invite you to book a demo of pharmazie.com’s platform today. See firsthand how consolidating OTC and Rx drug data for Germany in one place can transform your workflows and empower you to make informed, confident decisions. Your journey in the German pharmaceutical market will be smoother, faster, and more efficient with the right data partner – and pharmazie.com is here to support you every step of the way.
Take the next step toward data-driven success in Germany’s pharma market – schedule your personal pharmazie.com demo now and unlock the full potential of a truly comprehensive OTC and Rx drug database in Germany.