Germany SPCs ROTE LISTE in a nutshell
The GERMAN SPCs ROTE LISTE® provides detailed information in following aspects:
- Legal data to the German Drugs
- Short Products´Characteristics (SPCs)
With the GERMAN SPCs ROTE LISTE®, medical-pharmaceutical experts such as physicians, pharmacists, clinics, nursing staff, and pharmaceutical-technical assistants receive brief information on human finished medicinal products available in Germany and certain medical devices including EU approvals. These data are compiled from available specialist, usage and product information. In order to be included and published in the RED LISTE®, the pharmaceutical company must register its product or products accordingly. Without registration, the products are not mentioned. The Rote Liste® is a “trusted source” for drug information in Germany as listed by the European Medicines Agency (EMA) .
By licensing the GERMAN SPCs ROTE LISTE® is available in various databases, including via [pharmazie.com].
Suitable for:
Pharma Industry, Pharma Trade, Health Insurance, Regulatory
Source: Rote Liste Service LTD.
Frankfurt am Main, Germany
Related subscription
This database can be accessed after purchasing the subscription package ‘Drug Dictionaries’ or ‘Premium’
These databases could also be interesting for you
Related posts
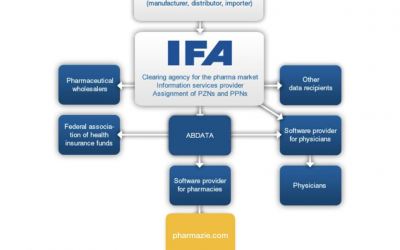
IFA Pharmaceuticals and their way into pharmaceutical databases
IFA Pharmaceuticals and their way into pharmaceutical databasesHow does the IFA medicines information get from the ABDA article master to pharmaceutical databases like pharmazie.com? This question is answered in the following article. You will learn who the IFA is,...
The Planetary Health Diet
The Planetary Health Diet 37 scientists from 16 countries have gazed into the future, and it will not work without our help! You can read in this article how exactly this diet and the rescue of the planet will work. Today we are confronted with an ever-increasing...